NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Multinodular goiter (MNG) is the most common of all the disorders of the thyroid gland. MNG is the result of the genetic heterogeneity of follicular cells and apparent acquisition of new cellular qualities that become inheritable. Nodular goiter is most often detected simply as a mass in the neck, but sometimes an enlarging gland produces pressure symptoms. Hyperthyroidism develops in a large proportion of MNGs after a few decades, frequently after iodine excess. Diagnosis is based on the physical examination. Thyroid function test results are normal, or indicate subclinical or overt hyperthyroidism. Imaging procedures are useful to detect details such as distortion of the trachea, and to provide an estimation of the volume before and after therapy. From 4 to 17% of MNGs fulfill the criteria of malignant change, however, the majority of these lesions are not lethal. If a clinical and biochemically euthyroid MNG is small and produces no symptoms, treatment is controversial. T4 given to shrink the gland or to prevent further growth is effective in about one third of patients. If the clinically euthyroid goiter is unsightly, shows subclinical hyperthyroidism or is causing pressure symptoms, treatment with ¹³¹I preceded by recombinant human TSH is successful but causes hypothyroidism in varying degrees. This treatment can lead to 45-65% shrinkage of the MNG, even if in an intrathoracic position, with a relatively low cost, thus it is considered a good alternative to surgery. However, surgery is an acceptable option. The efficacy of T4 treatment after surgery, to prevent regrowth, is debatable although frequently usedt. For complete coverage of all related aeas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
The normal thyroid gland is a fairly homogenous structure, but nodules often
form within its substance. These nodules may be only the growth and fusion of localized colloid-filled follicles, or more or less discrete adenomas, or cysts. Nodules larger than 1 cm may be detected clinically by palpation. Careful examination discloses their presence in at least 4% of the general population. Nodules less than 1 cm in diameter not clinically detectable unless located on the surface of the gland, are much more frequent. The terms adenomatous goiter, nontoxic nodular goiter, and colloid nodular goiter are used interchangeably as descriptive terms when a multinodular goiter is found.
INCIDENCE
The incidence of goiter, diffuse and nodular, is very much dependent on the status of iodine intake of the population. In areas of iodine deficiency, goiter prevalence may be very high and especially in goiters of longstanding, multinodularity develops frequently (Figure 17-1). The incidence of multinodular goiter in areas with sufficient iodine intake has been documented in several reports (1-10). In a comprehensive population survey of 2,749 persons in northern England, Tunbridge et al (1) found obvious goiters in 5.9% with a female/male ratio of 13:1. Single and multiple thyroid nodules were found in 0.8% of men and 5.3% of women, with an increased frequency in women over 45 years of age. Routine autopsy surveys and the use of sensitive imaging techniques produce a much higher incidence. In three reports nodularity was found in 30% to 50% of subjects in autopsy studies, and in 16% to 67% in prospective studies of randomly selected subjects on ultrasound (2). In Framingham the prevalence of multinodular goiter as found in a population study of 5234 persons over 60 years was 1% (3). Results from Singapore show a prevalence of 2.8% (4). In an evaluation in 2,829 subjects, living in southwestern Utah and Nevada (USA, between 31 and 38 years) of age, 23% had non-toxic goiter, including 18 single nodules, 3 cysts, 38 colloid goiters and 7 without a histological diagnosis. No mention was made of multinodular goiters, although some might have been present in the colloid and unidentified group (5). In general, in iodine sufficient countries the prevalence of multinodular goiter is not higher than 4% (6). In countries with previous deficiency that was corrected by universal salt iodination, elderly subjects may have an incidence of, approximately, 10% of nodular and multinodular goiter, attributed to lack of nutritional iodine in early adult life (7).
ETIOLOGY
The first comprehensive theory about the development of multinodular goiter was proposed by David Marine (8) and studied further by Selwyn Taylor (9), and can be considered one of the classics in this field. Nodular goiter may be the result of any chronic low-grade, intermittent stimulus to thyroid hyperplasia. Supporting evidence for this view is circumstantial. David Marine first developed the concept, that in response to iodide deficiency, the thyroid first goes through a period of hyperplasia as a consequence of the resulting TSH stimulation, but eventually, possibly because of iodide repletion or a decreased requirement for thyroid hormone, enters a resting phase characterized by colloid storage and the histologic picture of a colloid goiter. Marine believed that repetition of these two phases of the cycle would eventually result in the formation of nontoxic multinodular goiter (8). Studies by Taylor of thyroid glands removed at surgery led him to believe that the initial lesion is diffuse hyperplasia, but that with time discrete nodules develop (9).
By the time the goiter is well developed, serum TSH levels and TSH production rates are usually normal or even suppressed (10). For example, Dige-Petersen and Hummer evaluated basal and TRH-stimulated serum TSH levels in 15 patients with diffuse goiter and 47 patients with nodular goiter (11). They found impairment of TRH-induced TSH release in 27% of the patients with nodular goiter, suggesting thyroid autonomy, but in only 1 of the 15 with diffuse goiter. Smeulers et al (12), studied clinically euthyroid women with multinodular goiter and found that there was an inverse relationship between the increment of TSH after administration of TRH, and size of the thyroid gland (Figure 17-1). It was also found that, while being still within the normal range, the mean serum T3 concentration of the group with impaired TSH secretion was significantly higher than the normal mean, whereas the mean value of serum T4 levels was not elevated (12). These and other results (13) are consistent with the hypothesis that a diffuse goiter may precede the development of nodules. They are also consistent with the clinical observation that, with time, autonomy may occur, with suppression of TSH release, even though such goiters were originally TSH dependent.
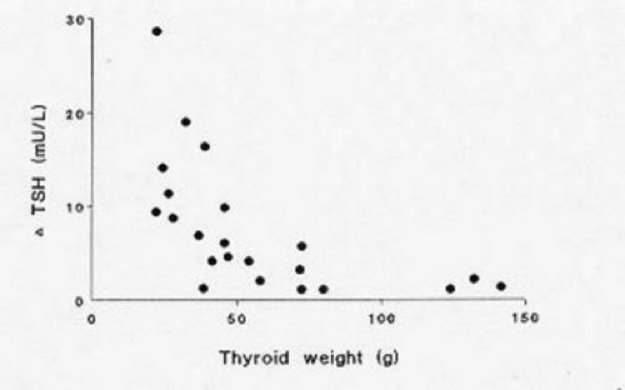
Figure 17-1
Relationship of TSH (after 400 mg TRH i.v.) and thyroid weight (g) in 22 women with clinically euthyroid multinodular goiter (with permission ref. 12)
Comprehensive reviews about insights into the evolution of multinodular goiter have been published by Studer and co-workers (14-16). An adapted summary of the major factors that are discussed is presented in Table 17-1 and will be referred to in the discussion that follows.
Table 17-1. Factors that may be involved in the evolution of multinodular goiter.
PRIMARY FACTORS
- Functional heterogeneity of normal follicular cells, most probably due to genetic and acquisition of new inheritable qualities by replicating epithelial cells. Gender (women) is an important factor.
- Subsequent functional and structural abnormalities in growing goiters.
SECONDARY FACTORS
- Elevated TSH (induced by iodine deficiency, natural goitrogens, inborn errors of thyroid hormone synthesis)
- Smoking, stress, certain drugs
- Other thyroid-stimulating factors (IGF-1 and others)
- Endogenous factor (gender)
PRIMARY FACTORS
Genetic heterogeneity of normal follicular cells and acquisition of new inheritable qualities by replicating epithelial cells. (Figure 17-2).It has been shown cells of many organs, including, the thyroid gland, are often polyclonal, rather than monoclonal of origin. Also from a functional aspect it appears that through developmental processes the thyroid epithelial cells forming a follicle are functionally polyclonal and possess widely differing qualities regarding the different biochemical steps leading to growth and to thyroid hormone synthesis like e.g. iodine uptake (and transport), thyroglobulin production and iodination, iodotyrosine coupling, endocytosis and dehalogenation. As a consequence there is some heterogeneity of growth and function within a thyroid and even within a follicle Studer et al (14-16) demonstrated the existence of monoclonal and polyclonal nodules in the same multinodular gland. They analyzed 25 nodules from 9 multinodular goiters and found 9 to be polyclonal and 16 monoclonal. Three goiters contained only polyclonal nodules and 3 contained only monoclonal nodules. In 3 goiters poly- and monoclonal nodules coexisted in the same gland (17).

Figure 17-2
Heterogeneity of morphology and function in a human multinodular goiter. Autoradiographs of two different areas of typical multindular euthyroid human goiter excised after administration of radioiodine tracer to the patient. There are enormous differences of size, shape and function among the individual follicles of the same goiter. Note also that there is no correlation between the size or any other morphological hallmark of a single follicle and its iodine uptake. (with permission ref.15).
Newly generated cells may acquire qualities not previously present in mother cells. These qualities could subsequently be passed on to further generations of cells. A possible example of this process is the acquired abnormal growth pattern that is reproduced when a tissue sample is transplanted into a nude mouse (16). Other examples are acquired variable responsiveness to TSH (13). These changes may be related to mutations in oncogenes which do not produce malignancy per se, but that can alter growth and function. An example of acquisition of genetic qualities is the identification in the last few years of constitutively activating somatic mutations not only in solitary toxic adenoma, but also in hyperfunctioning nodules of toxic multinodular goiters (18). So far these mutations in MNG have only been found in the TSH-receptor (TSHR) gene, and not in the Gs-alpha gene. Different somatic mutations are found in exon 9 and 10 of the TSHR gene and the majority of mutations that are present in toxic adenomas are also found in toxic nodules in multinodular goiter (19-21).
Genes associated with multinodular goiter
In contrast to sporadic goiters, caused by spontaneous recessive genomic variation, most cases of familial goiter present an autosomal dominant pattern of inheritance, indicating predominant genetic defects. Gene-gene interactions or various polygenic mechanisms (i.e. synergistic effects of several variants or polymorphisms) could increase the complexity of the pathogenesis of nontoxic goiter and offer an explanation for its genetic heterogeneity (22-26). A strong genetic predisposition is indicated by family and twin studies (27-29). Thus, children of parents with goiter have a significantly higher risk of developing goiter compared with children of nongoitrous parents (24). The high incidence in females and the higher concordance in monozygotic than in dizygotic twins also suggested a genetic predisposition (24). Moreover, there is preliminary evidence of a positive family history for thyroid diseases in those who have postoperative relapse of goiter, which can occur from months to years after surgery.
Defects in genes that play an important role in thyroid physiology and thyroid hormone synthesis could predispose to the development of goiter, especially in case of borderline or overt iodine deficiency. Such defects could lead to dyshormonogenesis as an immediate response, thereby indirectly explaining the nodular transformation of the thyroid as late consequences of dyshormonogenesis, as a form of maladaptation (12). The genes that encode the proteins involved in thyroid hormone synthesis, such as the thyroglobulin-gene (TG-gene), the thyroid peroxidase-gene (TPO-gene), the sodium – iodide – symporter-gene (SLC5A5), the Pendred syndrome-gene (SLC26A4), the TSH receptor-gene (TSH-R-gene), the iodotyrosine deiodinase (DEHAL 1) and the thyroid oxidase 2 gene3 (DUOX2) are convincing candidate genes in familial euthyroid goiter (30). Originally, several mutations in these genes were identified in patients with congenital hypothyroidism (30). However, in cases of less severe functional impairment, with can still be compensated, a contribution of variants of these genes in the etiology of nontoxic goiter is possible.
Linkage studies
A genome-wide linkage analysis has identified a candidate locus, MNG1 on chromosome 14q31, in a large Canadian family with 18 affected individuals (31). This locus was confirmed in a German family with recurrent euthyroid goiters (32). A dominant pattern of inheritance with high penetrance was assumed in both investigations. Moreover, a region on 14q31 between MNG1 and the TSH-R-gene was identified as a potential positional candidate region for nontoxic goiter (33). However, in an earlier study the TSH-R-gene was clearly excluded (31). Furthermore, an X-linked autosomal dominant pattern and linkage to a second locus MNG2 (Xp22) was identified in an Italian pedigree with nontoxic familial goiter (34). To identify further candidate regions, the first extended genome-wide linkage analysis was performed to detect susceptibility loci in 18 Danish, German and Slovakian euthyroid goiter families (35). Assuming genetic heterogeneity and a dominant pattern of inheritance, four novel candidate loci on chromosomes 2q, 3p, 7q and 8p (36) were identified . An individual contribution was attributable to four families for the 3p locus and to 1 family to each of the other loci, respectively. On the basis of the previously identified candidate regions and the established environmental factors, nontoxic goiter can consequently be defined as a complex disease. However, for this first time a more prevalent putative locus, present in 20% of the families investigated, was identified (35).
The candidate region on 3p (37) suggests a dominant pattern of inheritance for goiter. However, whereas linkage studies are suitable for the detection of candidate genes with a strong effect it is possible to miss weak genetic defects of first-line candidate gene-variants or of novel genes by linkage studies. Moreover, it is conceivable that the sum of several weak genetic variations in different genomic regions could lead to goiter predisposition. Therefore, the widely accepted risk factors such as iodine deficiency, smoking, old age, and female gender are likely to interact with and / or trigger the genetic susceptibility (22).
Mutagenesis leading to multinodular goiter
Most goiters become nodular with time. (Figure 17-3) From animal models of hyperplasia caused by iodine depletion (38) we have learned that besides an increase in functional activity a tremendous increase in thyroid cell number occurs. These two events likely induce a number of mutation events. It is known that thyroid hormone synthesis goes along with increased H2O2 production and free radical formation with may damage genomic DNA and cause mutations. Together with a higher spontaneous mutation rate, a higher replication rate will more often prevent mutation repair and increase the mutation load of the thyroid, thereby also randomly affecting genes essential for thyrocyte physiology. Mutations that confer a growth advantage (e.g. TSH-R mutations) very likely initiate focal growth. Hence, autonomously functioning thyroid nodules (AFTNs) are likely to develop from small cell clones that contain advantageous mutation as shown for the TSH-R in “hot” microscopic regions of euthyroid MNG (18).
Epidemiologic studies, animal models and molecular/genetic data outline a general theory of nodular transformation. Based on the identification of somatic mutations and the predominant clonal origine of AFTNs and cold thyroid nodules (CTNs) the following sequence of events could lead to thyroid nodular transformation in three steps. First, iodine deficiency, nutritional goitrogens or autoimmunity cause diffuse thyroid hyperplasia (39-41). Secondly, at this stage of thyroid hyperplasia, increased proliferation together with a possible DNA damage due to H2O2 action causes a higher mutation load, i.e. a higher number of cells bearing mutations. Some of these spontaneous mutations confer constitutive activation of the cAMP cascade (e.g. TSH-R mutations) which stimulates growth and function. Finally, in a proliferating thyroid, growth factor expression (e.g. insulin-like growth factor 1 [IGF-1], transforming growth factor ß [TGF-ß], or epidermal growth factor [EGF]) is increased (42-51). As a result of growth factor co-stimulation most cells divide and form small clones. After increased growth factor expression ceases, small clones with activating mutations will further proliferate if they can achieve self-stimulation. They could thus form small foci, which could develop into thyroid nodules. This mechanism could explain AFTNs by advantageous mutations that both initiate growth and function of the affected thyroid cells as well as CTNs by mutations that stimulate proliferation only. Moreover, nodular transformation of thyroid tissue due to TSH secreting pituitary adenomas, nodular transformation of thyroid tissue in Graves´ disease and in goiters of patients with acromegaly could follow a similar mechanism, because thyroid pathology in these patients is characterized by early thyroid hyperplasia.
As an alternative to the increase of cells mass, and as illustrated by those individuals who do not develop a goiter when exposed to iodine deficiency, the thyroid might also adapt to iodine deficiency without extended hyperplasia. Although the mechanism that allows this adaptation is poorly understood, data from a mouse model suggests an increase of mRNA expression of TSH-R, NIS and TPO in response to iodine deficiency, which might be a sign of increased iodine turnover in the thyroid cell in iodine deficiency. Moreover, expansion of the thyroid microvasculature, caused by up regulation of vascular endothelial growth factor and other proangiogenic factors, could be an additional mechanism that might help the thyroid to adapt to iodine deficiency (52).
SECONDARY FACTORS
The secondary factors discussed below stimulate thyroid cell growth and / or function and, because of differences in cellular responsiveness that are presumed to exist, aggravate the expression of heterogeneity which leads to further growth and focal autonomic function of the thyroid gland. Local necrosis, cyst formation sometimes with bleeding and fibrosis may be the anatomical end stage of such processes (Figure 17-3).

Figure 17-3
Mild iodine deficiency associated or not with smoking, presence of natural goitrogenic, drugs, familial goiter, genetic markers and gender (women) will decrease the inhibition of serum T4 on the pituitary thyrotrophs. Increased TSH production will cause diffuse goiter followed by nodule formation. Finally, after decades of life, a large multinodular goiter is present with cystic areas, hemorrhage, fibrosis and calcium deposits.
Iodine Deficiency
Stimulation of new follicle generation seems to be necessary in the formation of simple goiter. (Figure 17-3) Evidence accumulated from many studies indicates that iodine deficiency or impairment of iodine metabolism by the thyroid gland, perhaps due to congenital biochemical defects, may be an important mechanism leading to increases in TSH secretion (30,53). Since in experimental animals the level of iodine per se may modulate the response of thyroid cells to TSH, this is an additional mechanism by which relatively small increases in serum TSH level may cause substantial effects on thyroid growth in iodine-deficient areas (53). It was found that the thyroidal iodine clearance of patients with nontoxic nodular goiter was, on overage, higher than that in normal persons (Fig. 17-3). This finding was interpreted as a reflection of a suboptimal iodine intake by such patients. When data published from various major cities in Western Europe, regarding thyroid volume and iodine excretion are put together (54) and inverse relation is found between urinary iodine excretion and thyroid volume (Fig. 17-4). Physiologic stresses, such as pregnancy, may increase the need for iodine and require thyroid hypertrophy to increase iodine uptake that might otherwise satisfy minimal needs. An elevated renal clearance of iodine occurs during normal pregnancy (24). It has been suggested that in some patients with endemic goiter there are similar increases in renal iodine losses (53). Increased need for thyroxin during pregnancy may also lead to thyroid hypertrophy when iodine intake as limited. Iodide need in pregnancy is increased by increased iodide loss through the kidneys, but also because of significant transfer of thyroid hormone from the mother to the fetus (24). In areas of moderate iodine intake, thyroid volume increase is predominantly affected by a higher HCG serum concentration during the first trimester of pregnancy, and by a slightly elevated serum TSH level present at delivery (24). Finally mutations in the thyroglobulin gene may impair the efficiency of thyroid hormone synthesis and release, leading to a decreased rate of inhibition of TSH at pituitary level. The relatively high TSH released from the thyrotrophs will continuously stimulate the thyroid gland growth (55).
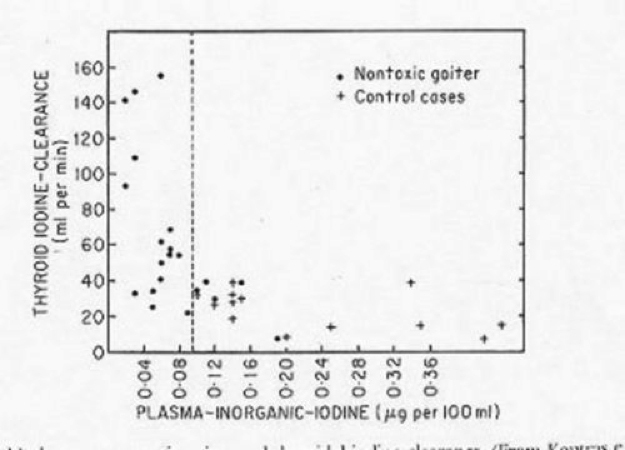
Figure 17-4Relationship between nontoxic goiter and thyroidal iodine clearance.
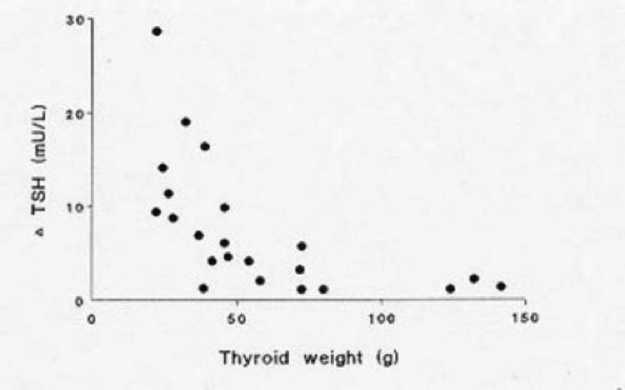
Figure 17-5Correlation between thyroid volume and urinary iodine excretion in normal population from various areas.
Natural occurring goitrogens
Patients occasionally have thyroid enlargement either because of goitrogenic substances in their diet or because of drugs that have been given for other conditions (53). Feeding rats with minute doses of a natural goitrogen over many months will result in the same kind of response. Similar results have been obtained using combinations of the three most prevalent goitrogens contained in cabbage. The explanation for the effect of such substances is that the goitrogen is much more effective at the level of iodothyronine synthesis than at earlier steps in hormone production such as iodide trapping. Thus, the RAIU may be high, but with a block in hormone synthesis the stage would be set for the production of a goiter. This possibility remains to be proved in humans, but one might surmise that, if true, it would operate most effectively in a situation of borderline iodine supply. The goitrogen thiocyanate potentiates the effect of severe iodine deficiency in endemic areas of Africa (53).
Several natural occurring goitrogens are listed in Table 17-2. Note that excessive
Nutritional use of seaweed (rich in iodine) may induce goiter. Moreover malnutrition (protein-caloric malnutrition) iron deficiency, selenium deficiency when associated with marginally low nutritional iodine may impair thyroid hormone synthesis and induce thyroid enlargement.
Table 17-2Natural goitrogens associated with Multinodular Goiter
| Goitrogens | Agent | Action |
|---|---|---|
| Millet, soy beans | Flavonoids | Impairs thyroperoxidase |
| Cassava, sweet potato, sorghum | Cyanogenic glucosides metabolized to thiocyanates | Inhibits iodine thyroidal uptake |
| Babassu coconut | Flavoniods | Inhibits thyroperoxidase |
| Cruciferous vegetables: Cabbage, cauliflower, Broccoli, turnips | Glucosinolates | Impairs iodine thyroidal uptake |
| Seaweed (kelp) | Iodine excess | Inhibits release of thyroidal Hormones |
| Malnutrition, Iron deficiency | Vitamin A deficiency Iron deficiency | Increases TSH stimulation Reduces heme-dependent thyroperoxidase thyroidal activity |
| Selenium | Selenium deficiency | Accumulates peroxidase and cause deiodinase deficiency; impairs thyroid hormone synthesis |
Modified and adapted from Medeiros-Neto & Knobel, ref. 53 | ||
Inherited defects in thyroid hormone synthesis and resistance to thyroid hormone action
Inherited goiter and congenital hypothyroidism were first described by Stanbury and associated (30) in two goitrous siblings with defective thyroperoxidase action resulting in impaired iodine organification. Both siblings were mentally retarded and had enormous multinodular goiters. In the next fifty years a number of genetic defects in every step of thyroid hormone synthesis have been described in detail. If not diagnosed at birth the impaired thyroid hormone synthesis would result in an elevated TSH secretion and diffuse goiter could progressively appears. Other factors might be of importance regarding goiter formation. The level of nutritional iodine seems to be quite important in patients with the defective sodium iodine symporter (NIS), thyroglobulin gene mutations and the defective dehalogenase system (DEHAL gene). If a relatively high intake of iodine is provided goiter formation may be slowed down to a certain extent. On the contrary in marginally low nutritional iodine intake goiter will progress to a very large size and nodules will appear (multinodular goiter). It has been proposed that mutations of certain genes involved in thyroid hormone synthesis that do not entirely affect the physiological action of the translated protein may cause goiter later on life and more frequently in women (55). Thus the variable phenotype resulting from genetically documented mutations may be quite variable depending on environmental factors (iodine). Individual adaptation to the defective protein, rapid hydrolysis of defective TG, serum level of TSH and response of the thyroid epithelial cells to the growth-promoting effect of TSH are other factors to be considered.
It is conceivable that multinodular goiter could result from a defect in any step of thyroid hormone synthesis, and to resistance to thyroid hormone action. In both groups of defects in the thyroid hormone system serum TSH would be elevated and goiter would be the logical consequence of a prolonged stimulation to growth. In the context of other factor that might induce multinodular goiters the defective thyroid hormone system and resistance to thyroid hormone action are relatively rare conditions as compared to other factors.

IOD Iodine organification defect; PIOD: Partial IOD;TIOD: Total IOD; RAI: radioactive iodine. Source: modified from “Genetic causes of dyshormonogenesis. Grasberger and Refetoff, 2011.
Other Thyroid-Stimulating Factors
Other substances that could be involved in stimulating thyroid enlargement are epidermal growth factor (EGF) and insulin-like growth factors (IGF). EGF stimulates the proliferation of thyrocytes from sheep, dogs, pigs, calves, and humans (42-51). While stimulating growth, EGF reduces trapping and organification of iodide, TSH receptor binding, and release of thyroglobulin, T3 and T4. On the other hand TSH may modulate EGF binding, to thyroid cell membranes and thyroid hormone may stimulate EGF production and EGF receptor number. In a study on adenomatous tissue, obtained from patients with multinodular goiter, it was found, by immunohistochemistry, that expression of EGF was increased (43). IGF-2 interacts with trophic hormones to stimulate cell proliferation and differentiation in a variety of cell types. The interaction between TSH and IGF-2 is synergestic (44). Increased IGF-I expression may contribute to goiter formation. A similar synergistic effect may exist between IGF-I and TSH. This synergism on DNA synthesis is mediated by complex interactions including the secretion of one or more autocrine amplification factors. Non-functioning nodules in patients with multinodular goiter contain the same IGF-1 receptors that are present in the normal adjacent extra-nodular follicles but are expressed in higher concentrations. Fibroblast growth factor (FGF)-1, stimulates colloid accumulation in thyroids of rat s but only in the presence of TSH (43). Expression of FGF-1 and -2 and FGF-receptor-1 will be followed by thyroid hyperplasia and may play a role in development of multinodular goiter (49). Fancia et al (50) found that in goiters with aneuploid components growth rate was higher than when euploid components were present (51). Other factors promoting cell growth and differentiation have been identified in the past. These include cytokines, acetylcholine, norepinephrine, prostaglandins, substances of neural origin like vasoactive intestinal peptide, and substances of C-cell origin. It is however not known to what extent these compounds play a role in the genesis of multinodular goiter.
The hypothesis that the development of thyroid autonomy is due to a gradual increase in the numbers of cells having relatively autonomous thyroid hormone synthesis is supported by the 27% prevalence of impaired TSH responses to TRH in patients with nodular goiter as opposed to such responses in only 1 of 15 patients with diffuse goiter (11). Such partial autonomy may appear only with time and could possibly be prevented by TSH-suppressive therapy. The fact that it is possible to induce hyperthyroidism in some patients with multinodular goiters by administration of iodide suggests that certain of the nodules in the multinodular gland are autonomous but unable under normal iodine intake to concentrate sufficient quantities of iodide to cause hyperthyroidism (53). Presumably iodide administration provides sufficient substrate for generation of excessive amounts of hormone, although it does not readily account for the long persistence of the hyperthyroidism in some of those cases.
Thus, there may be several etiologic factors in simple and nodular goiter, and some of these factors may act synergistically. The end result is a collection of heterogeneously functioning thyroid follicles, some of which may be autonomous and produce sufficient amounts of thyroid hormone to cause hyperthyroidism.
PATHOLOGY
Although it is rare to obtain pathological examination of thyroid glands in the early phase of development of multinodular goiters, such glands should show areas of hyperplasia with considerable variation in follicle size. The more typical specimen coming to pathologists is the goiter that has developed a nodular consistency. Such goiters characteristically present a variegated appearance, with the normal homogeneous parenchymal structure deformed by the presence of nodules (Figure 17-6). The nodules may vary considerably in size (from a few millimeters to several centimeters); in outline (from sharp encapsulation in adenomas to poorly defined margination for ordinary nodules); and in architecture (from the solid follicular adenomas to the gelatinous, colloid-rich nodules or degenerative cystic structures). The graphic term “Puddingstone goiter” has been applied. Frequently the nodules have degenerated and a cyst has formed, with evidence of old or recent hemorrhage, and the cyst wall may have become calcified. Often there is extensive fibrosis, and calcium may also be deposited in these septae. Scattered between the nodules are areas of normal thyroid tissue, and often-focal areas of lymphocytic infiltration. Radioautography shows a variegated appearance, with RAI localized sometimes in the adenomas and sometimes in the paranodular tissue. Occasionally, most of the radioactivity is confined to a few nodules that seem to dominate the metabolic activity of the gland.
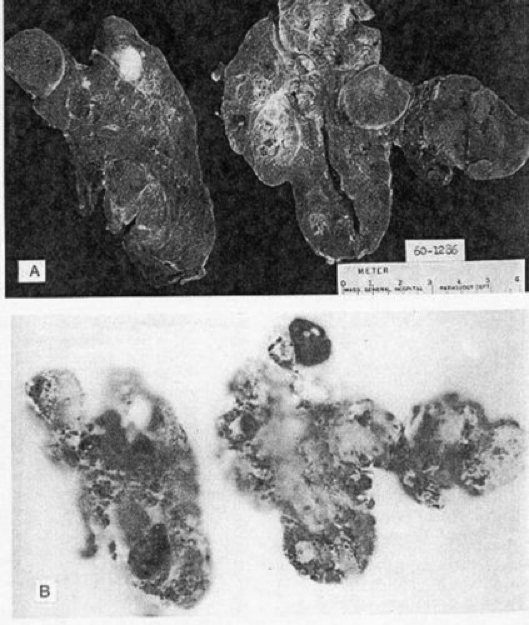
Figure 17-6
(A) Cross section of multinodular goiter. (B) Cross radioautograph of
The thyroid in part a. Observe the variation in 131I uptake indifferent areas.
If careful sections are made of numerous areas, 4-17% of these glands removed at surgery will be found to harbor microscopic papillary carcinoma (56-60). The variable incidence can most likely be attributed to the different criteria used by the pathologists and the basis of selection of the patients for operation by their physicians. These factors are discussed below.
NATURAL HISTORY OF THE DISEASE
Multinodular goiter is probably a lifelong condition that has its inception in adolescence or at puberty. Minimal diffuse enlargement of the thyroid gland is found in many teenage boys and girls, and is almost a physiologic response to the complex structural and hormonal changes occurring at this time. It usually regresses, but occasionally (much more commonly in girls) it persists and undergoes further growth during pregnancy. This course of events has not been documented as well as might be desired in sporadic nodular goiter, but it is the usual evolution in areas where mild endemic goiter is found.
Patients with multinodular goiter seek medical attention for many reasons. Perhaps most commonly they consult a physician because a lump has been discovered in the neck, or because a growth spurt has been observed in a goiter known to be present for a long time. Sometimes the increase in the size of the goiter will cause pressure symptoms, such as difficulty in swallowing, cough, respiratory distress, or the feeling of a lump in the throat. Rarely, an area of particularly asymmetrical enlargement may impinge upon or stretch the recurrent laryngeal nerve. Commonly the goiter is discovered by a physician in the course of an examination for some other condition. An important scenario is for the patient to seek medical attention because of cardiac irregularities or congestive heart failure, which proves to be the result of slowly developing thyrotoxicosis. (The issue is discussed more fully later in this chapter). Many times the goiter grows gradually for a period of a few too many years, and then becomes stable with little tendency for further growth. It is rare for any noteworthy spontaneous reduction in the size of the thyroid gland to occur, but patients often describe fluctuation in the size of the goiters and the symptoms they give. These are usually subjective occurrences, and more often than not the physician is unable to corroborate the changes that the patient describes. On the other hand, it could be that changes in blood flow through the enlarged gland account for the symptoms.
Occasionally, a sudden increase in the size of the gland is associated with sharp pain and tenderness in one area. This event suggests hemorrhage into a nodular cyst of the goiter, which can be confirmed by ultrasound. Within 3-4 days the symptoms subside, and within 2-3 weeks the gland may revert to its previous dimensions. In such a situation, acute thyrotoxicosis may develop and subside spontaneously.
Rarely, if ever, do the patients become hypothyroid and if they do, the diagnosis is more probably Hashimoto´s thyroiditis than nodular goiter. In a study in clinically euthyroid subjects with multinodular goiter, 13 out of 22 had subnormal TSH release after TRH. (12) If the goiter is present for long time, thyrotoxicosis develops in a large number of patients. In a series collected many years ago at the Mayo Clinic, 60% of patients with MNG over 60 were thyrotoxic. The average duration of the goiter before the onset of thyrotoxicosis was 17 years; the longer the goiter had been present the greater was the tendency for thyrotoxicosis to develop. This condition appears to occur because with the passage of time, autonomous function of the nodules develops. In a study of patients with euthyroid multinodular goiter, thyroid function was autonomous in 64 and normal in 26. After a mean follow-up of 5.0 years (maximum 12 years) 18 patients with autonomous thyroid function became overtly hyperthyroid and in 6 patients with primarily normal thyroid function autonomy developed (25-26). Thyroid function tests is illustrated in a patient with multinodular goiter starting from complete euthyroidism on to overt thyrotoxicosis. Occasionally a single discrete nodule in the thyroid gland becomes sufficiently active to cause thyrotoxicosis and to suppress the activity of the rest of the gland. (see Chap13). If these patients are given thyroid hormone, continued function of nodules can be demonstrated by radioiodine scanning techniques. Thus, these nodules have become independent of pituitary control. When patients with euthyroid multinodular goiter are frequently tested, it appears that in some of them occasional transient increases of serum T3 and / or T4 are seen. The possibility that the abrupt development of hyperthyroidism may follow administration of large amounts of iodine to these patients was reviewed by Stanbury and collaboration (61). In several areas of the world previously iodine deficiency the introduction of iodine supplementation lead to an increase of hyperthyroidism (non-autoimmune) possibly by excessive thyroid hormone production by “hot” thyroid nodules.
MULTINODULAR GOITER AND CANCER
If surgical specimens of multinodular goiters are examined carefully, 4-17% are found to harbor a carcinoma (56-60, 62-64). The use of ultrasound-guided fine needle aspiration (FNA) for evaluating these patients is not clearly defined. The biopsy of all the numerous nodules is impractical. Recently, a retrospective study with 134 patients showed a significant incidence (46,3%) of thyroid cancer in patients with multinodular goiter and benign FNA (65).These carcinomas vary widely in size and are typically of the papillary variety. Similar tumors are occasionally found in thyroid glands affected by Hashimoto´s thyroiditis and in otherwise normal glands. Bisi et al (59) reported that 13% of the glands resected in thyroid operations for any reason contained papillary adenocarcinoma. In Japan, routine autopsies of patients who were not suspected of having thyroid disease and who had no known irradiation experience, 17% were found to have small carcinomas when careful serial sections of the thyroid glands were done (62). If the figures of Bisi et al (59) were confirmed (63, 64) truly represent the prevalence of invasive carcinoma, one would certainly be forced to conclude that all multinodular goiters should be resected in order to prevent dissemination of malignant disease. However, it seems quite unlikely that all lesions that appear to satisfy the histological criteria for malignant neoplasia are potentially lethal. This view is strongly supported by the final report of the study on the significance of nodular goiter carried out in Framingham (see ref. 24). They followed for 15 years all 218 nontoxic thyroid nodules previously detected in a total population of approximately 5,000 persons. None of these lesions showed any clinical evidence of malignancy at the end of that time. Despite of the low-quality, the evidence suggests a lower prevalence of thyroid caner in multinodular goiter compared to single nodules, particularly in iodine-deficient areas (66, 67).
A strong case can be made for the view that there is only minimal risk from carcinoma in multinodular goiter. The prevalence of clinical nodularity of the thyroid is at least 4%, or 40,000 per 1,000,000 populations. Use of a much higher figure can be justified by the autopsy studies described above. Despite the high frequency of nodular goiter, only 36-60 thyroid tumors appear per 1,000,000 persons each year or by analysis of reported statistics on thyroid surgical specimens (57-60). A recent national cancer survey in the United States found an incidence of 40 per 1,000,000. An overview of the incidence of thyroid cancer in 409 countries, both with and free of endemic goiter was reported previously (58). The range of incidence varied between 7.5 and 56 per 1,000,000 persons each year. The prevalence of significant thyroid carcinoma at routine autopsy is less than 0.1% and persons with this type of tumor are probably examined as frequently as are those with other forms of neoplasia. The United States mortality figures for thyroid carcinoma are constant at about 6 per 10-6 population each year. Riccabona also summarized death rates from thyroid cancer in non-endemic and in endemic countries. (64) For Austria this was 16 per 10-6 per year in 1952 and 10 per 10-6 per year in 1983. For Switzerland this was in 1952, 18 per 10-6 per year and in 1979, 9 per 10-6 per year. The death rate per year for the United States in 1979 was 3 per 10-6, for Israel in 1952 1 per 10-6 per year and for the UK 7 per 10-6 in 1963. Death rates from thyroid cancer in endemic goiter areas from regions in Austria, Yugoslavia, Finland and Israel were between 10 and 16 per 10-6 per year between 1980 and 1984.
Lastly, it should be recognized that meticulous examination of autopsy specimens from persons dying of nonthyroid disease may show small (less than 0.5 cm) papillary lesions in4-24% of human thyroid glands (63,64). A report of 1020 sequential autopsies revealed the presence of microscopic papillary carcinoma in 6%. (60) Although the prevalence of this type of lesion increases with age, there is no question that such lesions may be present even in younger persons. The proportion of these lesions that even become clinically apparent is unknown, but their presence in otherwise normal thyroid glands should be kept in mind when evaluating reports of similar prevalences of thyroid carcinoma in multinodular thyroid glands.
If 4% of patients with nodular goiter actually have thyroid carcinoma, the prevalence of tumor in the general population would be 1,600 per 1,000,000. It is remarkable that only about 25 of these 1,600 hypothetical tumors would become apparent each year, or that only about 10 would prove fatal. Thus, there appears to be a gross discrepancy between the mortality form thyroid carcinoma and its reported frequency in surgical specimens of multinodular goiters. Reasonable arguments can be mustered in an effort to reconcile the information. Perhaps the most important single factor is selection. Persons with nodular goiter who come to operation are not representative of the general population but are patients with clinically significant thyroid disease who have been selected by their physicians for thyroid surgery. One of the factors controlling the selection process is the suspicion of malignant tumor. In fact, the selection process is especially good, as reflected by the high recovery of malignant thyroid tumors in patients operated on with this presumptive diagnosis. A second factor is that the histologic diagnosis of thyroid carcinoma may not correlate well with true invasiveness. It is impossible to prove this thesis, but pathologists agree that the criteria for judging malignancy are variable and that it is exceedingly difficult to predict with any degree of certainty the growth potential of a particular thyroid lesion.
Other arguments may be used to defend a conservative therapeutic position. In the first place, the tumors that are usually found in multinodular goiters are papillary tumors, and their degree of invasiveness is low. Indeed, the survival rate for intrathyroid papillary carcinoma is only slightly less than that for normal persons of the same age and sex (69-74). Furthermore, prophylactic subtotal thyroidectomy is not a guarantee of protection from cancer arising in a nodular goiter, since the process is usually diffuse, and it may be assumed that abnormal tissue is left in the neck after operation. In fact, unless replacement therapy is given, partial thyroidectomy might be expected to induce a tremendous growth stimulus in the remaining gland (75-80). A further point is that thyroidectomy, even in the best of hands, carries its own risk and its own morbidity, with dimensions comparable to those of missing a small papillary carcinoma within a multinodular goiter (81-84). Obviously this last possibility does not apply when a focus of unusual induration or rapid growth rate is detected clinically.
Diagnosis
Many of the symptoms of multinodular goiter have already been described. They are chiefly due to the presence of an enlarging mass in the neck and its impingement upon the adjacent structures. There may be dysphagia, cough, and hoarseness. Paralysis of recurrent laryngeal nerve may occur when the nerve is stretched taut across the surface of an expanding goiter, but this event is very unusual. When unilateral vocal cord paralysis is demonstrated, the presumptive diagnosis is cancer. Pressure on the superior sympathetic ganglions and nerves may produce a Horner´s syndrome.
As the gland grows it characteristically enlarges the neck, but frequently the growth occurs in a downward direction, producing a substernal goiter. A history sometimes given by an older patient that a goiter once present in the neck has disappeared may mean that it has fallen down into the upper mediastinum, where its upper limits can be felt by careful deep palpation. Hemorrhage into this goiter can produce acute tracheal obstruction. Sometime substernal goiters are attached only by a fibrous band to the goiter in the neck and extend downward to the arch of the aorta. They have even been observed as deep in the mediastinum as the diaphragm. Occasionally the skilled physician can detect a substernal goiter by percussion, particularly if there is a hint from tracheal deviation, or the presence of a nodular mass in the neck above the manubrial notch.
Symptoms suggesting constriction of the trachea are frequent, and displacement of the trachea is commonly found on physical examination. Computer Tomography examination is useful in defining the extent of tracheal deviation and compression. Compression is frequently seen but rarely is functionally significant have expected to find softened tracheal cartilage after the removal of some large goiters, but tracheomalacia has been observed only on the rarest occasion. Patients may be remarkably tolerant of nodular goiter even when the enlargement is striking. This finding is especially true in the endemic goiter areas of the world.
It is generally agreed that, thyroid isotope or ultrasound scanning are of little or no use in the diagnosis of carcinoma in a multinodular goiter. Two aspects are important in the differentiation from malignancy. First, the clinical presentation, if the goiter is of longstanding, showing little or no growth, absence of a dominant node, familial, while there is no neck irradiation in the past, especially in childhood, no hoarse voice, and no suspicious lymphnodes in the neck, there is little fear for carcinoma.
Table 17-4Clinical symptoms and investigations in the diagnosis of MNG
Simptoms and signs
Often family history of benign thyroid disease
Slowly growing anterior neck mass
Uni- or multinodularity on examination
Enlargment during pregnancy
Cosmetic complaints
Asymmetry, tracheal deviation, and/or compression
Rarelly upper airway obstruction, dyspnea, cough, and dysphagia
Sudden transient pain or enlargement secondary to hemorrhage
Gradually developing hyperthyroidism
Superior vena cava obstruction syndrome (rare)
Recurrent nerve palsy (rare)
Horner´s syndrome (rare)
Investigations
TSH normal or decreased, normal free T4, and free T3,
Serum Tg usually elevated
Thyroid autoantibodies (TPO and Tg) usually negative
Scintigraphy with solitary or multiple hot and/or cold areas
Ultrasound finding of solitary or multiple nodules with varying
echogenicity (nonhomogeneity)
Computed tomography and MR imaging demonstrating solitary or
multiple nodules with varying echogenicity
Lung function testing may demonstrate impaired inspiratory capacity
Fine-needle aspiration of solitary or dominant nodules – benign cytology
Modified and adapted from Hegedus et al (24)
Laboratory investigation
The choice of tests to investigate the functional status of a patient with a Simple diffuse goiter or Multinodular goiter may differ depending on the geographic areas of the world. Recent surveys conducted in the American, European and Latin American Thyroid Associations have indicated that the North American thyroidologists are quite restrictive in the choice of laboratory tests. Most of the experts, however, would perform a serum TSH and serum Free T4 test. In other settings Total T4 and Total T3 are also included because of the preferential secretion of T3 over T4 in mild iodine deficiency (53).
Antibodies against thyro-peroxidase (anti-TPO) and thyroglobulin (anti-TG) are measured, routinely, by most Europeans and Latin Americans thyroidologists. This seems to be relevant because thyroid auto antibodies are found approximately in 10% of the population and, consequently, autoimmunity may coexist with a goiter. Also diffuse or focal lymphocytic infiltration in an enlarged gland may represent chronic autoimmune thyroiditis.
Although serum TG correlates with the iodine status and the size of the enlarged thyroid gland it has little or no value in the diagnosis of goiter.
Diagnostic imaging
Neck palpation is notoriously imprecise with regard to thyroid morphology and size estimation (85). Several imaging methods are available in most settings: scintilography (with radioiodine, technetium), ultrasonography, computed tomography scans, magnetic resonance imaging and, less frequently used, positron emission tomography (PET). In Table 17-5 it is listed the characteristics, advantages and disadvantages of these imaging methods.
Ultrasonography of the thyroid
The main reasons for the widespread use of thyroid sonography are availability (several portable models are widely available at a relatively affordable price), the low cost of the procedure (if performed in the office or in the thyroid clinic), limited discomfort for the patient, and the non ionizing nature of the method. Ultrasonography may detect non palpable nodules cysts, will estimate nodule and goiter size (volume), will monitor the changes following therapy and will guide the Fine Needle Aspiration Biopsy (FNAB). After the introduction of ultrasonography it has become clear that nodules in the thyroid gland are very prevalent, ranging from 17% to 60% if older people are included in the study (85-95).
Hypoechogenicity, micro-calcifications, indistinct borders, increased nodular flow (visualized by DOPPLER) may have predictive value in distinguishing malignant from benign nodules (even in Multinodular Goiters).
The possibility of measuring thyroid volume is another highly useful feature of ultrasonographic studies particularly after therapy with L-T4 or radioiodine ablation. The volume of the goiter is usually based on the ellipsoid method (length, width depth X pi/6). This has an observer coefficient of variation of more than 10%. When compared to CT planimetry the ellipsoid method underestimate the goiter volume by 20%. Ultrasonography can not evaluate a multinodular goiter that has partially migrated to the upper mediastinum.
Ultrasound elastography can also provide information regarding malignant risk of thyroid nodules and multinodular goiter, however with questionable sensitivity (75%) and specificity (45,73%) (96).
Scintigraphy (isotope imaging)
It was used routinely in the past but at present has little place in the evaluation of a multinodular goiter (97-101). It is helpful in the determination of the functionality of the various nodules of a MNG. Thyroid scintigrams have been used through many years for measurement of the thyroid volume but compared to other methods is very inaccurate (24).
Computed tomography (CT) and Magnetic resonance (MR)
CT and MR provide high-resolution visualization of the goiter (Simple diffuse, multinodular). The major strength of CT and MR is their ability to diagnose and assess the extent of subesternal goiters (Fig. 17-7). Another advantage of the CT is the possibility for planimetric volume estimations, quite useful in irregularly enlarged multinodular goiter (102-105).
Recently the ionizing radiation delivered by a CT procedure has been source of concern for both clinicians and radiologists. Therefore the use of CT as an imaging method should be reserved for intra thoracic multinodular goiters, with tracheal compression.
Table 17-5Characteristics of imaging procedures in relation to nodular thyroid disease
| Advantages | Disadvantages | |
| Sonography | · High Availability · High morphologic resolution · No ionizing irradiation · Dynamic picture · Blood flow visualization (Doppler) · Biopsy guidance, also of lymph nodes · Moderate precision in volume estimation | · Operator dependency · No information of functionality · Not feasible in substernal goiter · Poor prediction of malignancy |
| Scintigraphy | · Information of functionality · Differentiates between destructive and hyperthyroid conditions · Measurement of thyroid iodine uptake · Predictive of feasibility of ¹³¹I therapy · Detects ectopic thyroid tissue | · Requires nuclear medicine · Ionizing irradiation · Poor resolution · Poor differentiation between solid and cystic cold nodules · Volume estimationinaccurate |
| CT Scan | · High morphologic resolution · Visualization of adjacent structure · Ideal for substernal goiter · Planimetric volume estimation · Volume estimation probably accurate | · Ionizing irradiation · No information of functionality · Poor prediction of malignancy |
| MR imaging | · No ionizing irradiation · High morphologic resolution · Visualization of adjacent structure · Ideal for substernal goiter · Planimetric volume estimation · Volume estimation with high precision | · Moderate availability · Long procedure time · Not usable with metallic objects inside patient · No information of functionality · Poor prediction of malignancy |
| PET | · Information of functionality · Metabolic investigations · Good prediction of malignancy | · Low availability and high cost · Requires specialized units · Ionizing irradiation |
CT, Computed tomography. MR, magnetic resonance Modified and adapted from Hegedus et al (24) | ||
Treatment of multinodular goiter
In the past iodine supplementation seems to be an adequate approach because goiter development is associated with mild iodine deficiency in many countries worldwide. The effect of iodine once a multinodular goiter has developed a limited value in reducting the MNG. A major problem of iodine supplementation is the risk for inducing subclinical / clinical hyperthyroidism (Jod-Basedow). Therefore aside from a few European Countries iodine is no longer used alone or associated with L-T4 to treat thyroid enlargement (24).
This leaves in essence three modalities of therapy:
(1). L-T4 suppressive therapy
(2). Radioiodine (¹³¹I) alone or preceded by rhTSH
(3). Surgery
L-T4 suppressive therapy
L-T4 suppressive therapy is used extensively both in Europe, USA and Latin America, according to their respective surveys. A beneficial effect of L-T4 has been demonstrated in diffuse goiters in many controlled trials (106-112). A goiter reduction of 20-40% can be expected in 3-6 months of therapy, the goiter returning to the pre-treatment size after L-T4 withdrawal. The efficacy of L-T4 is shown to depend on the degree of TSH suppression. When it comes to the nontoxic MNG there are five controlled studies in which sonography was used for objective size monitoring. Berghout et al (113) in a randomized double-blind trial showed that the goiter volume was reduced by 15% (9 months of L-T4 therapy). In the placebo group the goiter continued to increase in size by more than 20% in the 9 months period. The goiter volume returned to baseline values after discontinuation of the therapy. Lima et al (109) studied 62 patients with nodular goiter. Thirty per cent of patients were regarded as responders (reduction > 50% of the initial volume). In the control group 87% showed no change or an increase in goiter size. Wesche et al (110) compared L-T4 with ¹³¹I therapy in a randomized trial. The median reduction of goiter volume in the radioiodine treated group was 38-44% whereas only 7% of the L-T4 treated patients had a significant goiter reduction.
Papini et al (111) treated 83 goitrous patients (nodular goiter) with suppressive doses of L-T4 comparing the results with a control group. The L-T4 therapy was extended for 5 years. There was a decrease in nodular size in the L-T4 treated group and a mean volume increase in the control group. After 5 years sonograms detected 28.5% new nodules in the control group but only 7.5% in the L-T4 treated group. In conclusion long term TSH suppression induced volume reduction in a subgroup of thyroid nodules but effectively prevented the appearance of new nodules.
Zelmanovitz et al (112) studied 42 women with a single colloid nodule. Twenty one patients were treated with 2.7µg/kg of L-T4 for one year. Six of the 21 treated patients had a >50% reduction of the nodule volume as evaluated by sonography as compared to only 2 (out of 24 patients) that received placebo. They concluded that L-T4 therapy is associated with 17% of reduction of a single colloid nodule and may inhibit growth in other patients. They also conducted a meta-analysis of 6 prospective controlled trials and concluded that four of seven studies favors treatment with L-T4. The treatment of single nodules or multinodular goiter with L-T4 is an open issue as the reduction of the nodule / MNG is only obtained in about one third of patients. The possible unwanted effects of L-T4 therapy have also to be considered (114, 115).
Table 17-6Controlled studies of L-T4 therapy in multinodular goiter using a
precise thyroid size determination
| Authors (Country) | (n) | Duration of
L-T4 therapy | Dose of L-T4 | Outcome of continuous L-T4 | Therapy vs. Controls |
| Berghout et al (The Netherlands) | 55 | 9 months | 2.5μg/kg | 25% reduction among responders* | 20% had Increase of nodular volume |
| Lima et al (Brazil) | 62 | 12 months | 200μg/dia | 30% reduction** | No variation volume |
| Wesche et al (The Netherlands) | 57 | 24 months | 2.5μg/kg | 22% reduction | 44% volume with Radioidine |
| Papini et al (Italy) | 83 | 5 years | 2,0μg/kg 7.5% new nodules | 47.6% reduction 28.5% new nodules | 22% had reduction nodules |
| Zelmanovitz et al (Brazil) | 45 | 12 months | 2.7μg/kg | 28% reduction** | 8.3% had reduction |
| (*) Effective response to L-T4 therapy: volume was reduced by 13% of basal (**) Effective response to L-T4 therapy: volume reduction >50% of basal | |||||
Radioiodine ablation of goiter
General considerations: It has long been recognized that radioiodine administration results in shrinkage of the goitrous thyroid gland. Over 20 years ago ¹³¹I therapy reduced the MNG volume by approximately 40% in the first year, and 50-60% in the second year. In very large goiters with volume over 100 mL the reduction is less (around 35%). Patient with substernal MNG have also been treated with beneficial results. The individual response to radioiodine therapy, regarding goiter reduction and development of hypothyroidism is very difficult to predict. Goiter reduction is related to the absorbed thyroid dose. In most centers ¹³¹I doses of 3.7 MBq/g of thyroid tissue corrected for 100% 24h radioiodine uptake have been given. In other centers a fixed doses of radioiodine (100mCi, 150mCi) are administered according to the thyroid volume. The risk of permanent hypothyroidism after ¹³¹I therapy in MNG ranges from 11 to 58% after 1 to 8 years of follow-up (116-124).
The use of rhTSH for improving ¹³¹I therapy of nontoxic multinodular goiter
(1). Increased uptake and goiter volume reduction
In recent years, pretreatment with rhTSH has been used in patients with MNG (which typically have only a fraction of the normal RAIU) to increase ¹³¹I uptake in the goiter and allow treatment with lower doses of ¹³¹I to induce thyroid volume reduction (125-129). Accordingly, in a study of 15 patients with nontoxic MNG, pretreatment with a single low dose of rhTSH (0.01 or 0.03 mg 24 h before ¹³¹I administration) resulted in a doubling of RAIU (130). In addition, the single dose of rhTSH caused a more homogeneous distribution of ¹³¹I by stimulating more uptake in relatively cold areas than in hot areas, particularly in patients with low serum TSH levels (Figure 17- 7).
Various studies have demonstrated the effect of rhTSH on ¹³¹I therapy for MNG. Twenty-two patients with MNG were treated with ¹³¹I 24h after administration of 0.01 or 0.03 mg rhTSH (131). In this study, the dose of ¹³¹I was adjusted to the increase in uptake induced by rhTSH, aimed at 100 µCi/g thyroid tissue retained at 24h. Pretreatment with 0.01 and 0.03 mg rhTSH resulted in reductions in the ¹³¹I dose by a factor of 1.9 and 2.4, respectively. One year after treatment, there was a reduction in thyroid volume of 35% and 41% in the two groups, respectively. Despite delivering a good therapeutic response, the administration of ¹³¹I 100 µCi/g of thyroid tissue corrected for 24-h RAIU raises concerns of irradiation of the surrounding neck structures and potential risk for stomach, bladder, and breast cancer, which have been reported after ¹³¹I therapy for toxic nodular goiter (24). In another study (132), 16 patients with MNG were treated with a fixed dose of ¹³¹I (30 mCi) 72h after pretreatment with 0.3 mg rhTSH, or 24h after pretreatment with 0.9 mg rhTSH. The two regimens were equally effective, leading to a 30 to 40% reduction in thyroidal volume at 3 to 7 months. Giusti et al compared the 12-months outcome after RAI and rhTSH arbitrarily chosen (0.1mg for 24-h RAIU > 30 %; 0.2 mg for RAIU<30 %) between patients with basal non-toxic (TSH>0.3 mIU/l)) and non autimmune pre-toxic MNG (TSH<0.3 mIU/l). They confirmed the effectiveness of rhTSH adjuvant treatment in reducing thyroid volume after low RAI dose (<600 MBq) independently of the baseline TSH level. A more severe thyrotoxic phase after rhTSH was observed in patients with TSH<0.3 mIU/L, while L-T4 therapy was more frequently needed when initial TSH levels were > 0.3 mIU/l (133)
As mentioned, rhTSH was administered 24h before ¹³¹I therapy in most studies. However, results from a study published by Duick and Baskin (134, 135) suggested that the time interval may be even longer to achieve a maximum stimulation of the thyroid RAIU.
Recently in a phase II, single blinded, placebo-controlled study with 95 patients evaluating two low doses (0.01 and 0.03mg) of modified-release rhTSH, no statistical significant enhancement of thyroid volume reduction was achieved at three years follow-up (41% to 53%) . The modified-release rhTSH was developed to minimize the side effects related to thyroid hormone excess, (136).
(2). Tracheal compression and pulmonary function
Many elderly patients have significant intrathoracic extension of the MNG, which may cause tracheal compression with subsequent airflow reduction. Bonnema et al (137) evaluated upper airway obstruction by flow volume loops in 23 patients with very large goiter. In one third of the patients, there was impairment of the forced inspiratory flow at 50% of the vital capacity (FIF50%).The authors found a significant correlation between FIF50% and the smallest tracheal cross-sectional area. Reduction of the MNG volume after high dose of ¹³¹I had a remarkable effect in enlarging tracheal cross-sectional area and consequently improving inspiratory capacity in these patients.
(3). Transient hyperthyroidism after ¹³¹I ablation
Other studies using different doses of rhTSH and showing comparable RAIU increases with lower doses, demonstrated significant goiter reduction, but also transient hyperthyroidism after ¹³¹I therapy (131-144). A study in which 34 patients with large MNGs were randomized to receive ¹³¹I therapy (100 µCi/g of thyroid tissue) alone or following a single relatively high dose of rhTSH (0,45 mg) 24h before ¹³¹I administration, showed that patients who received rhTSH had transient elevations in thyroid hormone levels lasting a few weeks, a greater reduction in goiter size (60% vs. 40%), and a higher incidence of hypothyroidism (65% vs. 21%) (142). In another study, 18 patients received two 0.1 mg doses of rhTSH followed by 30 mCi of ¹³¹I. RAIU increased from 12 to 55%, free T 4 increased from 1.3 to 3.2 ng/dL, and goiter size reduced from 97 to 65 mL. However, about 30% of the patients experienced painful thyroiditis and 39% had mild hyperthyroidism (137). In a randomized trial of ¹³¹I treatment calculated to deliver a thyroidal absorbed dose of 100 Gy (10 mrads) and administered 24h after rhTSH (0.3 mg) or placebo, patients with MNG (mean goiter volume of 55 cm³) who received rhTSH had more symptoms of hyperthyroidism and neck pain during the first week after treatment, a greater reduction in goiter size (52% vs. 46%), and a higher frequency of hypothyroidism (62% vs. 11%) (145). Using a similar study design, Bonnema et al (141) compared the effects of rhTSH (0.3 mg) or placebo, followed by a maximum dose of ¹³¹I 100 mCi on goiter volume reduction in 29 patients with very large goiters (median volume of 160 mL). After 12 months, the median goiter volume (monitored by magnetic resonance imaging) was reduced by 34% in the placebo group and by 53% in the rhTSH group. In the placebo group, the goiter reduction correlated positively with the retained thyroidal ¹³¹I dose, whereas this relationship was absent in the rhTSH group. Adverse effects, mainly related to thyroid pain and cervical compression, were more frequent in the rhTSH group. At 12 months, goiter-related complaints were significantly reduced in both groups without any between-group difference. One patient in the placebo group and three patients in the rhTSH group developed hypothyroidism.
Recently, an uncontrolled study (140) demonstrated the effect of rhTSH (0.1 mg, single dose) followed by ¹³¹I 30mCi 24h later in 17 patients with MNG (mean thyroid volume of 106 cm³). Pretreatment with rhTSH resulted in a mean RAIU increase from 18 to 50% and an increase in free T4 of 55% at 24h. Mean T3 levels increased by 86% and peaked at 48h, and median TG levels increased about 600% and peaked on the fifth day. Symptomatic tachycardia was promptly relieved with ß-blocker administration. After 12 months, mean thyroid volume measured by computed tomography had reduced by 46%. The adverse effects observed were transient hyperthyroidism (17.6%), painful thyroiditis (29.4%), and hypothyroidism (52.9%).
(4). Degree of goiter reduction, ¹³¹I dose, and rhTSH
Most investigators (Table 17-7) could not find any correlation of thyroid volume reduction with post-rhTSH RAIU, area under the curve of TSH, basal thyroid volume, or effective activity of ¹³¹I. Also, in the placebo-controlled study by Bonnema et al (141), no significant correlation was found, in either the placebo group of the rhTSH-treated group, between the degree of goiter reduction and the initial goiter size. However, in the placebo group, there was a correlation (r = 0.74) between the degree of goiter reduction and the retained ¹³¹I thyroid dose, an observation in agreement with previous reports (135). At variance, Albino et al (131) found a positive correlation (r = 0.68) between the degree of goiter volume reduction and the effective activity of administered post-rhTSH ¹³¹I dose. This issue, therefore, needs further clarification, but overall, these studies suggest that goiter reduction may be dependent on other factors caused by rhTSH pre-stimulation and not only on the applied ¹³¹I thyroid dose. For example, rhTSH could induce reactivation of inactive thyroid tissue or render the thyrocytes more vulnerable to ionizing radiation. Generally, the dose of ¹³¹I in these studies ranged from 75 to 400 µCi/g tissue, and most patients received doses between 100 and 200 µCi/g, similar to those used to treat hyperthyroidism.

Figure 17-7
– Goiter reduction volume (%) at last follow-up of patients treated only with radioiodine (left bars) as compared with patients that received recombinant human TSH plus radioiodine (right bars). Note the significant and early volume reduction with radioiodine preceded by rh TSH (146)
| Table 17-7. Studies on the effect of recombinant human TSH on goiter reduction | ||||||||||||||||
| in multinodular goiter patients. | ||||||||||||||||
| No. of subjects | Dose of rhTSH (mg) | Time interval between rhTSH and radioiodine (123I or 131I) | Therapeutic dose of 131I (mCi) | Peak increase in thyroid hormones (%) | Goiter reduction(%) | Time of follow-up | Goiter size estimation (Methods) |
Remarks
| ||||||||
| Nieuwlaat et al. (128) | 12 | 0.01 | 24 h | ~39 (mean) | Free T4: 47 Free T3: 41 | 35 | 1 year | MRI | 0.01 mg: 131I activity reduced by a factor 1.9 | |||||||
| 10 | 0.03 | 24 h | ~23 (mean) | Free T4: 52 Free T3: 59 | 41 | 0.03 mg: 131I activity reduced by a factor 2.4; Hypothyroidism: 36% | ||||||||||
| Duick & Baskin (134) | 6 | 0.3 | 72 h | 30 | NI | NI | 7 m | Palpation | 0.3 mg: increase in 4 h RAIU 72 h after rhTSH: from 3.9 to 17 | |||||||
| 10 | 0.9 | 24 h | 30 | NI | 30-40 | 0.9 mg: remission of compressive symptoms in 69% Hypothyroidism: 56% | ||||||||||
| Silva et al. (142) | 17 | none | ~96 (mean) | Free T4: 34 T3: 33 | 40 | 1 year | CT | 131I: Hypothyroidism: 23% | ||||||||
| 17 | 0.45 | 24 h | ~90 (mean) | Free T4: 594 T3: 73 | 58 | 131I + rhTSH: Hypothyroidism: 64%; hyperthyroidism: 100% | ||||||||||
| Albino et al. (131) | 18 | 2 x 0.1 | 24 h | 30 | Free T4: 146 T3: 191 | 39 | 6 m | CT | 24 h RAIU increased from 12 – 53%; Hypothyroidism: 65%; hyperthyroidism: 39% | |||||||
| Giusti et al. (140) | 8 | none | NM | NM | 25 | 20 m | US + CT | |||||||||
| 12 | 2x0.2 | 24 h | NM | Free T4: 290* Free T3: 340* | 44 | 22 m | US + CT | |||||||||
| Cohen et al. (132) | 17 | 0.03 | 24 h | ~30 | Free T4: 46 T3: 33 | 34 | 6 m | CT | 24 h RAIU increased from 26% to 43%; Hypothyroidism: 18%; hyperthyroidism: 18% | |||||||
| Nielsen et al. (145) | 29 | none | 14 (median) | NM | 46 | 1 year | US | 131I: 24 h RAIU decreased from 32 to 29; Hypothyroidism: 11%; hyperthyroidism: 21% | ||||||||
| 28 | 0.3 | 24 h | ~16 (median) | NM | 62 | 131I + rhTSH: 24 h RAIU increased from 34 to 47; Hypothyroidism: 62%; hyperthyroidism: 36% | ||||||||||
| Bonnema et al. (141) | 15 | none | 24 h | ~42 (median) | NM | 34 | 1 year | MRI | 131I: hypothyroidism: 7% | |||||||
| 14 | 0.3 | ~38 (median) | NM | 53 | 131I + rhTSH: hypothyroidism: 21% | |||||||||||
| Paz-Filho et al. (137) | 17 | 0.1 | 24 h | 30 | Free T4: 56 T3: 87 | 46 | 1 year | CT | 24 h RAIU increased from 18 to 50%; Hypothyroidism: 53%; hyperthyroidism: 18% | |||||||
| Cubas et al. (147) | 28 | A: 0.1 B: 0.005 C: NONE | 24 h | 30 | Free T4: 31 Free T4: 23 Free T4: 19 | 37.2 39.3 15.3 | 2 years | CT | 43% had hypothyroid signs 25.9% had persistant hypothyroidism | |||||||
| Romão et al. (148) | Eu: 18 SCH: 18 CH: 6 | 0.1 | 24h | 30 | Free T4: 67 Free T4: 106 Free T4: 170 | 79.5 70.6 68.7 | 3 years | CT | Hypothyroidism: 50% 11% 16% Side effects more commonly find in SCH and CH | |||||||
| Fast et al | Clontrol | 24h | rh TSH> | 1 year | see Figure 17-7 | |||||||||||
| (146) | Rh TSH | X control | ||||||||||||||
| Fast et al. (136) | 95 | Placebo 0.01 0.03 | 24hs | 100Gy | NM | 44 41 53 | 3 years | CT | Hypothyroidism: more frequently in 0.03 mg group | |||||||
| Giusti et al (133) | 26 | TSH>0.3mlU/l TSH<0.3mlU/l | 24hs | 600 MBq | NM | 67,1 61.7 | 55.3 ± 4.1m 57.2 ± 5.1m | Ultrasonography | Several side effects in both groups | |||||||
M: months
(5). Increase in goiter size immediately after ablation
It is worth mentioning the possibility of increase in goiter size with rhTSH (142, 145). In a study of 10 patients with MNG who were given 0.3 mg of rhTSH, it was shown that 24h after rhTSH, the mean goiter volume increased by 9.8% and after 48h, by 24%, reverting to baseline at 1 week. This suggests that rhTSH may lead to significant cervical compression in patients with near obstructive goiters, when used for improving ¹³¹I therapy in patients with goiter (145). All side effects related to acute thyroid enlargement causing tenderness and dyspnea due to possible obstruction of tracheal airway were promptly resolved with corticosteroid therapy.
(6). Radioactive iodine and rhTSH in elderly with hyperthyroidism
Treatment with ¹³¹I following rhTSH stimulation is also an attractive alternative in elderly patients considered poor surgical candidates or who refuse surgery. The prevalence of MNG rises in the elderly, a population in whom comorbities prevail. Of even greater concern in iodine repleted areas is the development of subclinical or overt hyperthyroidism, since thyroid hyper-function may increase the mortality risk in these patients (148). An Italian study assessed 20 elderly patients with large goiters and compared treatment with ¹³¹I (10 to 15 mCi fixed dose) following two consecutive 0.2 mg doses of rhTSH (n = 12; 3 patients had subclinical hyperthyroidism with TSH <0.3 µU/ml) with treatment with ¹³¹I alone (n = 8; subclinical hyperthyroidism recorded in 5). Patients who received rhTSH had higher transient elevations in free T4 and Free T3 lasting 2 weeks, a greater reduction in goiter size (44% vs. 25%). Both groups had a 17% incidence of hypothyroidism ~ 2 years after ¹³¹I therapy. Symptomatic relief occurred in all but 1 patient following rhTSH with a 50% median reduction on thyroid volume after about 2 years (140). In study conducted by Silva et al (142), 17 elderly subjects with MNG treatment with ¹³¹I 24h after pretreatment with 0.45 mg rhTSH and were compared with 17 elderly controls treated with ¹³¹I alone. In patients pretreated with rhTSH, serum TSH and T3 levels rose to a peak level in 24h, returning to normal at 72h. Serum free T4 concentrations rose significantly at 48h returning to normal at 7 days. Serum TG increased and remained elevated during the following 12 months. Patients pretreated with rhTSH had a 58% reduction in goiter volume when compared with 40% in patients treated with ¹³¹I alone. Hypothyroidism was more frequent in pretreated patients (65% versus 21% in non-pretreated) after 1 year. No symptoms of hyperthyroidism were observed in these patients. Four years after ¹³¹I therapy, additional thyroid volume reduction was similar for patients treated with rhTSH prior to ¹³¹I or with ¹³¹I alone, but it was significantly more pronounced in the rhTSH group, mainly in the first year (149). Although no additional benefit of rhTSH was observed after a long follow-up, the initial difference in thyroid volume reduction was maintained, denoting the advantage of using rhTSH pretreatment to achieve higher thyroid volume reduction during the first treatment (Table 17-6).
In another report of a short-term observational study, the investigators assessed the efficacy of a low-dose (0.03 mg) rhTSH stimulation on a fixed therapeutic activity of ~ 30 mCi ¹³¹I in 17 patients with large nodular goiters (12 with overt or subclinical hyperthyroidism / TSH <0.5 µU/ml and five on treatment with thionamides) (147). RAIU increased from 26% to 43%, free T4 increased from 1.4 to 2.0 ng/dl, and goiter size decreased from 170 to 113 cm³ by 6 months. Symptomatic relief, improved well-being and / or reduction, or elimination of anti-hyperthyroid drug was seen in 76% of the patients. However, 3 (18%) patients presented transient neck pain or tenderness, 1 experienced asymptomatic thyroid enlargement, and 3 became hypothyroid by 3 months (Table 17-6). A recent paper (146) compared the results of RAI alone and RAI preceded by rhTSH (see Figure 17-8) clearly demonstrating the efficacy of pre-treatment with rh TSH.
(7). Cardiovascular events after RAI ablation
Cardiovascular parameters to detect transient elevation of serum thyroid hormones were evaluated in 27 of 42 patients (age range 42-80 years) with large MNGs who were treated with rhTSH before receiving ¹³¹I 30 mCi (150). All patients presented a transient surge in serum levels of free T4 and total T3 into the hyperthyroid range following therapy. However, post-treatment cardiovascular evaluation did not show significant changes when compared with baseline evaluation, suggesting that treatment of MNGs with RAI after rhTSH stimulation does not affect structural and functional parameters of the heart. These findings are reassuring, particularly when considering treatment for older adults with comorbidities that preclude surgery.
(8). Thyroid autoantibodies occurrence after ¹³¹I therapy
Some studies have reported the development of thyroid antibodies associated with ¹³¹I therapy (151), however a direct cause-effect linking to rhTSH has not been demonstrated. These observations have been interpreted as an immunological response caused by the release of thyroid antigens from destroyed follicular cells. In a study published by Rubio et al (152), it was found that rhTSH pretreatment had no significant effect in the development of antibodies (TSH receptor and TPO) when compared with treatment with ¹³¹I alone. As noted below, up to 5% of individuals develop auto-immune hyperthyroidism after 131-I therapy.
(9). Potential induction of malignancy
Although generally ignored, treatment with large doses of 131-I obviously raises the possibility of induction of malignancy. This has not so far been recorded in relation to therapy of MNG. Depending on functionality of the thyroid tissue, dose administered, size of the goiter, and size of the patient, whole body radiation could be up to 1 rad/mCi given, a dosage similar to that obtained during therapy of thyroid cancer. Perhaps the major use of this treatment will be in older individuals, with a shorter potential life span after treatment, which would presumably make this less of a concern.
Conclusions and comments
Given the limited experience published in the literature so far, before considering the routine use of rhTSH administration before ¹³¹I treatment of MNG, several issues must be taken into consideration (153-157).
- ¹³³I treatment alone can lead to a 15-25% transient increase in thyroid volume during the first week after treatment;
- rhTSH administration alone occasionally can lead to a significant increase, albeit transient, in thyroid volume, of up to 100% in normal subjects with 48h.
- The combination of the two modalities may lead to a substantial acute increase in thyroid volume;
- ¹³¹I treatment of MNG leads to transient hyperthyroidism during the first 2-3 weeks after therapy and the combination with rhTSH administration can enhance this effect, with potential consequences particularly for the elderly patients (148);
- The optimal dose of rhTSH for pretreatment of MNG remains to be determined. Studies have used different doses and regimens or rhTSH administration, from as low as 0.01 or 0.03 mg to as high as 0.45 mg or 0.9 mg 24h before RAI treatment;
- There is a significant occurrence of hypothyroidism after ¹³¹I treatment following rhTSH stimulation;
- Although rare, autoimmune hyperthyroidism (approximate reported incidence of 4-5%) can develop after treatment of MNG with ¹³¹I;
- Currently, rhTSH is not approved by the FDA as an adjuvant for ¹³¹I treatment of goiter.
Based on these results, pretreatment with rhTSH seems a promising alternative to thyroid surgery for the management of nontoxic MNG, particularly in elderly individuals. However, the optimal dose and timing of both, rhTSH and ¹³¹I as well as the criteria for patient eligibility remain to be determined.
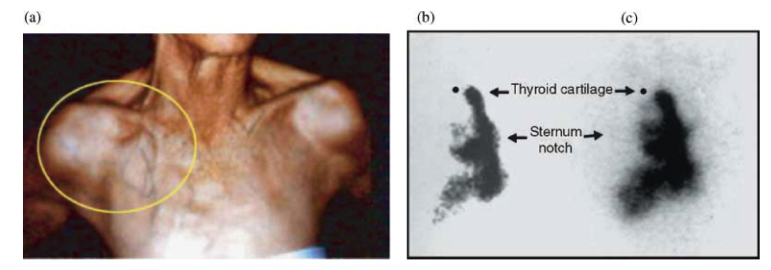
Surgery for MNG
As indicated by Fast et al (154) it is time to consider radioiodine treatment for MNG as an alternative to surgery. As indicated previously radioiodine (¹³¹I) is a simple, cost-effective and safe procedure with an impressive goiter reduction up to 65% of the original volume. Surgery of the MNG, however, is equally effective and the choice among the two procedures depends largely on their availability, clinical features, and last but not least the personal preference of the patient (and also the physician in charge). In many centers, specially in countries with large populations previously living in iodine deficiency, the number of patients with MNG, most of them, over 50 years old, are very common in the thyroid clinic daily routine. Therefore sending all those patients to surgery will inevitably, cause a logistic problem in terms of available surgical rooms, surgeons well trained in head and neck surgery, post surgical follow-up and all the costs involved. Moreover with the widespread use of ultrasonographic studies followed by Fine Needle Aspiration Biopsy (FNAB) the number of new cases of thyroid cancer has increased dramatically in the past few years. Obviously these patients will have precedence for a surgical therapy as compared with the patient with MNG. This situation is quite common in many countries where there is a long waiting list for a given patient to be selected for thyroid surgery. Frequently surgery of the thyroid due to a nodule harboring a papillary cancer in a relatively young subject has a definite preferential status over an elderly patient with a long standing MNG.
The preferred operation for MNG is subtotal thyroidectomy. The frequency of complications due to surgical depends on several factors and well-trained and experienced surgeons will reduce the rates of such complications. Recurrence after goiter surgery is rare and the frequency of hypothyroidism is low. It is advisable to introduce L-T4 therapy after surgery in order to avoid goiter recurrence although this option is considered highly controversial.
Laser Ablation Therapy
A retrospective study that assessed clinical records of 1534 benign nodules in 1531 patients treated with image-guided laser ablation therapy (LAT) showed that LAT induced a clinical relevant nodule volume reduction that ranges from 48 to 96% (72±11%) twelve months after treatment (158). This picture was more significantly in mixed nodules (range 70-92%, 79±7%). Most of the nodules (83%) received a single LAT dose while 13% received two doses and 3% three doses, with a total energy delivery based on the initial volume. The symptoms improved from 10% to 49% of cases (p<0.001), while major (voice changes) and minor complications (hematomas, skin burn) were rare. Thus, LAT is considered clinically effective and well-tolerated as an alternative to surgery for benign thyroid lesions.
To summarize: treatment of MNG with L-T4 suppressive doses is not accepted by many thyroidologists in spite of the fact that goiter reduction is achieved in one third of the patients and new nodules appearance is lower in the L-T4 treated patients.The results so far published using radioiodine preceded by rhTSH are quite encouraging . It is an excellent alternative when surgical teams are not available for all patients. LAT treatment should be performed only by experience operators. Finally, patients preference for a non- surgical alternative should always be taken into consideration.
SUMMARY
Perhaps the most common of all the disorders of the thyroid gland is multinodular goiter. Even in non-endemic regions it is clinically detected in about 4% of all adults beyond the age of 30. Pathologically it is much more frequent, the percentage incidence being roughly the same as the age of the group examined. The disease is much more common in women than in men.
Multinodular goiter is thought to be the result of primarily two factors. The first factor is genetic heterogeneity of follicular cells with regard to function (i.e. thyroid hormone synthesis) and growth. The second factor is the acquisition of new qualities that were not present in mother cells and become inheritable during further replication. Mutations may occur in follicular cells leading to constitutively activated adenomas and to hyperthyroidism. These factors may lead to loss of anatomical and functional integrity of the follicles and of the gland as a whole. These processes ultimately lead to goiter formation and are accelerated by stimulatory factors. These stimulatory factors are basically an elevated serum TSH, brought about by events such as iodine deficiency, inborn errors of thyroid hormone synthesis, goitrogens or local tissue growth-regulating factors. These basic and secondary factors may cause the thyroid to grow and gradually evolve into an organ containing hyperplastic islands of normal glandular elements, together with nodules and cysts of varied histologic pattern.
Nodular goiter is most often detected simply as a mass in the neck, but at times an enlarging gland produces pressure symptoms on the trachea and the esophagus. Occasionally tenderness and a sudden increase in size herald hemorrhage into a cyst. Hyperthyroidism develops in a large proportion of these goiters after a few decades frequently after iodine excess. Rare complications are paralysis of the recurrent laryngeal nerve, and pressure on the superior sympathetic ganglion causes a Horner´s syndrome.
The diagnosis is based on the physical examination. Thyroid function test results are normal or disclose subclinical or overt hyperthyroidism. Thyroid autoantibodies are usually absent or low, excluding Hashimoto´s thyroidits. Imaging procedures may reveal distortion of the trachea, calcified cysts, or impingement of the goiter on the esophagus. Sonographic studies, Scintilography (¹³¹I), CT and MRI are useful to detect details of the MNG and to provide an estimation of the volume before and after therapy.
From 4 to 17% of multinodular thyroids removed at operation contain foci that on microscopic examination fulfill the criteria of malignant change. The infrequency of thyroid cancer as a cause of death clearly proves that the vast majority of these lesions are not lethal or even clinically active. One of the reasons for the high incidence of cancer in surgical specimens is that patients with multinodular goiters were often selected for surgery because of a concern for carcinoma.
If a clinical and biochemically euthyroid multinodular goiter is small and produces no symptoms, treatment is controversial. T4 given in an effort to shrink the gland or to prevent further growth is effective in about one third of the patients. This therapy is more likely to be effective if begun at an early age while the goiter is still diffuse than in older patients in whom certain nodules may have already become autonomous. If the clinically euthyroid goiter is unsightly, shows subclinical hyperthyroidism or is causing, pressure symptoms, treatment with ¹³¹I preceded by recombinant human TSH is successful in virtually all cases but causes hypothyroidism at varying degree. Surgery is an acceptable alternative. The efficacy of T4 treatment after surgery, to prevent regrowth, is frequently used albeit debatable.
Overt toxic nodular goiter is usually treated with radioiodine. A gratifying reduction in the size of the goiter and control of the hyperthyroidism may be expected. Hypothyroidism often ensues.
During the past few years the use of recombinant human TSH has been used to enhance the uptake of radioiodine and to provide a more homogenous distribution of the radionuclide. Results have been rewarding with a 45-65% shrinkage of the MNG, even with an intrathoracic position. A surge of high levels of serum Free T4, total T3 and serum TG is observed in the first weeks after therapy. Clinically hyperthyroid patients seem to have more unwanted signs and symptoms as compared to euthyroid patients. Hypothyroidism (permanent) is commonly observed at 6-12 months after rhTSH plus RAI treatment. Taking all into account, this modality of treatment of MNG has a relatively low cost and it is considered a good alternative to surgery that might not be available for all patients with MNG in many centers around the world.
The term colloid is applied to glands composed of uniformly distended follicles appearing as a diffuse enlargement of the thyroid gland. The condition is found almost exclusively in young women. With time and due to a number of primary and secondary factors it may gradually develop into a multinodular goiter which becomes increasingly prominent as the decades pass. Appropriate therapy, if required, is the timely administration of thyroid hormone that may be continued for several years.
An intrathoracic goiter is usually an acquired rather than a development abnormality. It may come about in embryonic life by a carrying downward into the thorax of the developing thyroid anlage, or in adult life by protrusion of an enlarging thyroid through the superior thoracic inlet into the yielding mediastinal spaces. These lesions may produce pressure symptoms and may also be associated with hyperthyroidism. If too large for treatment with ¹³¹I, the appropriate therapy is resection of the goiter through the neck, if possible. Attachment of the intrathoracic goiter to the gland in the neck ordinarily proves the site of origin and provides a method for its easy surgical removal. In many of these patients a safe and easily performed therapy, in an outpatient mode, is the administration of a fixed dose of radioiodine (¹³¹I) of 30 mCi preceded by rhTSH.
REFERENCES
- Tunbridge WGM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG: The spectrum of thyroid disease in a community: The Whickham survey. Clin Endocrinol 7:481, 1977.
- Charib TGH, Thyroid incidentalomas: management approaches to non palpable nodules discovered incidentally on thyroid imaging. Ann Int Med 126:226-231, 1997.
- Sawin CT, Bigos ST, Land S, Bacharach P. The aging thyroid. Relationship between elevated thyrotropin levels and thyroid antibodies in elderly patients. Am J Med 79:591, 1985.
- Wang KW, Sum CF, Tan KT, Ng WY, Cheah JS, A study of non-toxic goiter. Ann Acad Med Singapore 19:439-442, 1990.
- Rallison ML, Dobyns BM, Meikle AW, et al. Natural history of thyroid abnormalities: prevalence, incidence, and regression of thyroid diseases in adolescents and young adults. Am J Med 91:363-370, 1991.
- Pinchera A, Aghini-Lombardi F, Antonangeli L, Vitti P. Multinodular goiter. Epidemiology and prevention. Ann Ital Chir 67:317-325, 1996.
- Jarlob AE, Nygaard B, Hegedus L, Hartling SC, Hansen JM: Oberver variation in the clinical and laboratory evaluation of patients with thyroid dysfunction and goiter. Thyroid 8:393-398, 1998.
- Marine D: Etiology and prevention of simple goiter. Medicine 3:453, 1924.
- Taylor S: The evolution of nodular goiter. J Clin Endocrinol Metab 13:1232, 1953.
- Beckers C, Cornette C: TSH production rate in nontoxic goiter. J Clin Endocrinol Metab 32:852, 1971.
- Dige-Petersen H, Hummer L: Serum thyrotropin concentrations under basal conditions and after stimulation with thyrotropin-releasing, hormone in idiopathic non-toxic goiter. J Clin Endocrinol Metab 44:1115, 1977.
- Smeulers J, Docter R, Visser TJ, Hennemann G: Response to thyrotrophin-releasing hormone and triiodothyronine suppressibility in euthyroid multinodular goiter. Clin Endocrinology 7:389, 1977.
- Berghout A, Wiersing WM, Smits NJ, Touber JL. Interrelationships between age, thyroid volume, thyroid nodularity, and thyroid function in patients with sporadic non-toxic goiter. Am J Med 89:602-608, 1995.
- Derwahl M, Studer H. Nodular goiter and goiter nodules: Where iodine deficiency falls short of explaining the facts. Exp Clin Endocrinol Diabetes 109:250-60, 2001.
- Studer H, Peter HJ, Gerber H: Natural heterogeneity of thyroid cells: The basis for understanding thyroid function and nodular growth. Endocr Rev 10:125, 1989.
- Peter JH, Gerber, Studer H, Smeds S: Pathogenesis of heterogeneity in human multinodular goiter. J Clin Invest 76:1992, 1985.
- Kopp P, Kimura ET, Aeschimann S, et al. Polyclonal and monoclonal nodules coexist with human multinodular goiters. J Clin Endocrinol Metab 79:134-139, 1994.
- Krohn K, Wohlgemuth S, Gerber H, Paschke R. Hot microscopic areas of iodine-deficient euthyroid goiters contain constitutively activating TSH receptor mutations. J Pathol 192:37-42, 2000.
- Holzapfel AP, Fuhrer D, Wonerow P, et al. Identification of constitutively activating somatic thyrotropin receptor mutations in a subset of toxic multinodular goiters. J Clin Endocrinol Metab 82:4292-4233, 1997.
- Tassi V, Di Cerbo A, Porcellini A, Papini E, Cisternino C, et al. Screening of thyrotropin receptor mutations by fine-needle aspiration biopsy in autonomous functioning thyroid nodules in multinodular goiters. Thyroid 9:353-357, 1999.
- Gabriel EM, Bergert ER, Grant CS, van Heerden JA, Thompson GB, et al. Germline polymorphism of codon 727 of human thyroid-stimulating receptor is associated with toxic multinodular goiter. J Clin Endocrinol Metab84:3328-3335, 1999.
- Krohn K, Führer D, Bayer Y, Eszlinger M, Brauer V, Neumann S, Paschke R. Molecular Pathogenesis of Euthyroid and Toxic Multinodular Goiter. Endocr Rev. 2005 June; 26(4):504-24.
- Masini-Repiso AM, Cobanillas AM, Bonaventura M, Coleoni AH. Dissociation of thyrotropin-dependent enzyme activities, reduced iodide transport, and preserved iodide organification in nonfunctioning adenoma and multinodular goiter. J Clin Endocrinol Metab 79:39, 1994.
- Hegedus L, Bonnema SJ, Bennedbek FN. Management of simple nodular goiter: current status and fature perspectives. Endocr Reviews 24:102-132, 2003.
- Krohn K, Paschke R. Progress in understanding the etiology of thyroid autonomy. J Clin Endocrinol Metab 86:3336-3345, 2001.
- Brix TH, Hededus L. Genetic and environmental factors in the aetiology of simple goiter. Ann Med 32:153-156, 2000.
- Brix TH, Kyvik KO, Hedegu¨s L. Major role of genes in the etiology of simple goiter in females: a population-based twin study. J Clin Endocrinol Metab 84:3071-3075, 1999.
- Brix TH, Kyvik KO, Hegedüs L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab 85:536-539, 2000.
- Medeiros-Neto G, Camargo RYC, Tomimori EK. Approach to and treatment of goiters. Med Clin N Am 92(2):351-358, 2012
- Knobel M & Medeiros-Neto G: An outline of inherited disorders of the thyroid hormone generating system. THYROID 13:771-801, 2003.
- Bignel GR, Canzian F, Shayeghi M, Stark M, Shugart YY, Biggs P, Mangion J, Hamoudi R, Rosenblatt J, Buu P, Sun S, Stoffer SS, Goldgar DE, Romeo G, Houlston RS, Narod SA, Stratton MR, Foulkes WD. Familial nontoxic multinodular thyroid goiter locus maps to chromosome 14q but does not account for familial nonmedullary thyroid cancer. Am J Hum Genet 61:1123-1130, 1997.
- Neumann S, Willgerodt H, Ackermann F, Reske A, Jung M, Reis A, Paschke R. Linkaged of familial euthyroid goiter to the multinodular goiter-1 locus and exclusion of the candidate genes thyroglobulin, thyroperoxidase and NA+/I- symporter. J Clin Endocrinol Metab 84:3750-3756, 1999.
- Mckay JD, Williamson J, Lesueur F, Stark M, Duffield A, Canzian F, Romeo G, Hoffman L. At least three genes account for familial papillary thyroid carcinoma: TCO and MNG1 excluded as susceptibility loci form a large Tasmanian family. Eur J Endocrinol 141:122-125, 1999.
- Capon F, Tacconelli A, Giardina E, Sciacchitano S, Bruno R, Tassi V, Trischitta V, Filetti S, Dallapiccola B, Novelli G. Mapping a dominant form of multinodular goiter to chromosome Xp22. Am J Hum Genet 67:1004-1007, 2000.
- Bayer Y, Neumann S, Meyer B. Genome-wide Linkage Analysis reveals evidence for four new susceptibility foci for familial euthyroid goiter. J Clin Endocr Metab 89:4044-53, 2004.
- Corval M, Perez R, Sanchez I, Mories MT, San Millan JL, Miralles JM, Gonzalez-Samiento R. Thyroglobulin gene point mutation associated with non-endemic simple goiter. Lancet 341:462-464, 1993.
- Takashi T, Nozaki JI, Komatsu M, Wada Y, Utsunomiya M, Inoue K, Takada G, Koisumi A. A new Locus for a dominant form of Multinodular Goiter on 3q26.1-q26.3 Bioch Biophy Res Comm 284:650-654, 2001.
- Samuels MH. Evaluation and treatment of sporadic nontoxic goiter-some answers and more questions. J Clin Endocrinol Metab 86:994-997, 2001.
- Bertelsen JB, Hegedüs L. Cigarette smoking and the thyroid. Thyroid 4:327-331, 1994.
- Brix TX, Hansen PS, Kyvik KO, Hegedüs L. Cigarette smoking and risk of clinically overt thyroid disease: a population-based twin case-control study. Arch Intern Med 160:661-666, 2000.
- Gaitan E. Environmental natural goitrogens. In: Peter F, Wiersinga WM, Hostalek U, eds. The thyroid and environment. New York: Schattauer; 69-78, 2000.
- Cheng YL. Birman KD, Schaudies RP, Ahmann AJ, d´Avis J, Geelhoed GW, Wartofsky L: Effects of epidermal growth factor on thyroglobulin and adenosine 3´,5´-monophosphate production by cultured human thyrocytes. J Clin Endocrinol Metab 69:771, 1989.
- Sugenoya A, Masuda H, Komatzu M, Jokojama S, Shimizu T, Fujimori M, Kobajashi S, Iida F. Adenomatous goitre: therapeutic strategy, postoperative outcome, and study of epidermal growth factor receptor. Brit J Surg 79:404, 1992.
- Maciel RM, Moses AC, Villone G, Tramontano D, Ingbar SH: Demonstration of the production and physiological role of insulin-like growth factor II in rat thyroid follicular cells in culture. J Clin Invest 82:1546, 1988.
- Phillips ID, Becks GP, Logan A, Wang JF, Smith C et al. Altered expression of insulin growth factor-1 (IGF-I) and IGF binding proteins during rat hyperplasia and involution. Growth Factors 10:207, 1994.
- Takahashi S-I, Conti M, Van Wyk JJ: Thyrotropin potentiation of insulin-like growth factor-I dependent deoxyribonucleic acid synthesis in FRTL-5 cells: Mediation by an autocrine amplification factor(s). Endocrinology 126:736, 1990.
- Vanelli GB, Barni T, Modigliani U, Paulin I, Serio M, Magi M, Fiorelli G, Balboni GC. Insulin-like growth factor-I receptors in non-functioning thyroid nodules. J Clin Endocrinol Metab 71:1175, 1990.
- deVito WJ, Chanoine J-P, Alex s, Fang S-L, Stone S. Effect of in vivo administration of recombinant acidic fibroblast growth factor on thyroid function in the rat: induction of colloid goiter. Endocrinology 131:729, 1992.
- Thompson SD, Franklyn JA, Watkinson JC, et al. Fibroblast growth factors 1 and 2 and fibroblast growth factor receptor 1 are elevated in thyroid hyperplasia. J Clin Endocrinol Metab 83:1336-1341, 1998.
- Francia FG, Azzolina L, Mantovani T, et al. Heterogeneity of nuclear DNA pattern and its relationship with cell cycle activity parameters in multinodular goiter. Clin Endocrinol 46:649-654, 1997.
- Bidey SP, Hill DJ, and Eggo MC. Growth factors and goitrogenesis. J Endocrinol 160:321-332, 1999.
- Gérard AC, Poncin S, Caetano B et al. Iodine deficiency induces a thyroid stimulating hormone-independent early phase of microvascular reshaping in the thyroid. Am J Pathol 172(3):748-60, 2008.
- Medeiros-Neto G and Knobel M. iodine deficiency disorders. In: deGroot LJ, Jameson JL, eds. Endocrinology 6th Ed. Chapter 88. New York, Elsevier, 2010.
- Hegedüs L. Thyroid size determined by ultrasound. Influence of physiological factors and non-thyroidal disease. Dan Med Bull 37:249-263.
- Rubio IGS, Medeiros-Neto G. Mutations of the thyroglobulin gene and its relevance to thyroid disorders. Curr Opin Endocrinol Diabetes Obes. 16(5):373-8, 2009.
- Pelizzo MR, Piotto A, Rubello D, Casara D, Fassina A, Busnardo B. High prevalence of occult papillary thyroid carcinoma in a surgical series for benign thyroid diseases. Tumori 76:255,1990.
- McCall A, Jarosz H, Lawrence AM, Paloyan E. The incidence of thyroid carcinoma in solitary cold nodules and in multinodular goiters. Surgery 100:1128, 1986.
- Koh KB, Chang KW. Carcinoma in multinodular goiter. Brit J Surg 79:266, 1992.
- Bisi H, Fernandes SO, Camargo RYA, Koch L, Abdo AH, Brito T. The prevalence of unsuspected thyroid pathology in 300 sequential autopsies with special reference to the incidental carcinoma. Cancer 64:1888-1893, 1989.
- Lang W, Borrusch H, Bauer L. Occult carcinomas of the thyroid. Evaluation of 1020 sequential autopsies. Am J Clin Pathol 90:72, 1988.
- Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, Vidor G, Braverman LE, and Medeiros Neto G. Iodine-induced hyperthyroidism: Occurrence and epidemiology. Thyroid 8(1):83-100, 1998.
- Fukunaga FH, Lockett LJ: Thyroid carcinoma in the Japanese in Hawaii. Arch Pathol Lab Med 92:6, 1971.
- Sampson RJ, Key CR, Buncher CR, Iijima S: Smallest form of papillary carcinoma of the thyroid. Arch Pathol Lab Med 91:334, 1971.
- Sampson RJ, Woolner LB, Bahn RC, Kurland LT: Occult thyroid carcinoma in Olmsted County, Minnesota: Prevalence at autopsy compared with that in Hiroshima and Nagasaki. Jpn Cancer 34:2072, 1974.
- Campbell MJ, Seib CD, Candell L, Gosnell JE, Duh QY, Clark OH, Shen WT. The underestimated risk of cancer in patients with multinodulargoiters after a benign fine needle aspiration. World J Surg. 39(3):695-700, 2015.
- Brito JP, Yarur AJ, Prokop LJ, McIver B, Murad MH, Montori VM. Prevalence of thyroid cancer in multinodulargoiter versus single nodule: a systematic review and meta-analysis. 23(4):449-55, 2013. Review.
- 67.
Pasqualetti G, Caraccio N, Basolo F, Miccoli P, Monzani F.Prevalence of thyroid cancer in multinodulargoiter versus single nodule: iodine intake and cancer phenotypes. 24(3):604-5, 2014
- 68.
Riccabona OA. Thyroid cancer: its epidemiology, clinical features and treatment. Springer Verlag 1987.
- 69.
Tomusch O, Machens A, Sekulla C, Ukkat J, Lippert H, Gastinger I, Dralle H. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg 24:1335-1341, 2000.
- 70.
Abdel Rahim AA, Ahmed ME, Hassan MA. Respiratory complications after thyroidectomy and the need for tracheostomy in patients with a large goiter. Br J Surg 86:88-90, 1999.
- 71.
Mariotti RA, Zannini P, Viani MP, Voci C, Pezzuoli G. Surgical treatment of substernal goiters. Int Surg 76:12-17, 1991.
- 72.
Torre G, Borgonovo G, Amato A, Arezzo A, Ansaldo G, De Negri A, Ughe M, Mattioli F. Surgical management of substernal goiter: analysis of 237 patients. Am Surg 61:826-831, 1995.
- 73.
Vadasz P, Kotsis L. Surgical aspects of 175 mediatinal goiters. Eur J Cardiothorac surg 14:393-397, 1998.
- 74.
Allo MD, Thompson NW. Rationale for the operative management of substernal goiters. Surgery 94:969-977, 1983.
- 75.
Röjdmark J, Järhult J. High long term recurrence rate after subtotal thyroidectomy for nodular goiter. Eur J Surg 161:725-727, 1995.
- 76.
Berghout A, Wiersinga WM, Drexhage HA, van Trotsenburg P, Smits NJ, van der Gaag RD, Touber JL. The long-term outcome of thyroidectomy for sporadic non-toxic goitre. Clin Endocr (Oxf) 31:193-199, 1989.
- 77.
Geerdsen JP, Frolund L. Thyroid function after surgical treatment of nontoxic goitre. A randomized study of postoperative thyroxine administration. Acta Med Scand 220:341-345, 1986.
- 78.
Bistrup C, Nielsen JD, Gregersen G, Franch P. Preventive effect of levothyroxine in patients operated for non-toxic goitre: a randomized trial of one hundred patients with nine years follow-up. Clin Endocrinol (Oxf) 40:323-327, 1986.
- 79.
Miccoli P, Antonelli A, Iacconi P, Alberti B, Gambuzza C, Baschieri L. Prospective, randomized, double-blind study about effectiveness of levothyroxine suppressive therapy in prevention of recurrence after operation: result at the third year of follow-up. Surgery 114:1097-1101, 1993.
- 80.
Feldkamp J, Seppl T, Becker A, Klisch A, Schlagheck R, Goretzki PE, Roher HD. Iodide or L-thyroxine to prevent recurrent goiter in an iodine-deficient area: prospective sonographic study. World J Surg 21:10-14, 1997.
- 81.
Seiler CA, Glaser C, Wagner HE. Thyroid gland surgery in an endemic region. World J Surg 20:593-596, 1996.
- 82.
Mishra A, Agarwal A, Agarwal G, Mishra SK. Total thyroidectomy for benign thyroid disorders in an endemic region. World J Surg 25:307-310, 2001.
- 83.
Pappalardo G, Guadalaxara A, Frattaroli FM, Illomei G, Falaschi P. Total compared with subtotal thyroidectomy in benign nodular disease: personal series and review of published reports. Eur J Surg 164:501-506, 1998.
- 84.
Hisham AN, Azlina AF, Aina EN, Sarojah A. Total thyroidectomy: the procedure of choice for multinodular goitre. Eur J Surg 167:403-405, 2001.
- 85.
Wiest PW, Hartshorne MF, Inskip PD, Crooks LA, Vela BS, Telepak RI, Williamson MR, Blumhardt R, Bauman JM, Tekkel M. Thyroid palpation vs high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med 17:487-496, 1998.
- 86.
Borghout A, Wiersinga WM, Smits NJ, Touber JL. The value of thyroid volume measured by ultrasonography in the diagnosis of goitre. Clin Endocrinol (Oxf) 28:409-414, 1988.
- 87.
Knudsen N, Bols B, Bülow I, Jorgensen T, Perrild H, Ovesen L, Laurberg P. Validation of ultrasonography of the thyroid gland for epidemiological purposes. Thyroid 9:1069-1074, 1999.
- 88.
Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med 154:1838-1840, 1994.
- 89.
Tomimori E, Pedrinola F, Cavaliere H, Knobel M, Medeiros-Neto G. Prevalence of incidental thyroid disease in a relatively low iodine intake area. Thyroid 5:273-276, 1995.
- 90.
Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC. [Volumetric analysis of thyroid lobes by real-time ultrasound.] Dtsch Med Wochenschr 106:1338-1340, 1981.
- 91.
Reinartz P, Sabri O, Zimmy M, Nowak B, Cremerius U, Setani K, Bull U. Thyroid volume measurement in patients prior to radioiodine therapy: comparison between three-dimensional magnetic resonance imaging and ultrasonography. Thyroid 12:713-717, 2002.
- 92.
Hegedus L, Perrild H, Poulsen LR, Andersen JR, Holm B, Schnohr P, Jensen G, Hansen JM. The determination of thyroid volume by ultrasound and its relationship to body weight, age, and sex in normal subjects. J Clin Endocrinol Metab 56:260-263, 1983.
- 93.
Nygaard B, Nygaard T, Court-Payen, Jensen LI, Soe-Jensen P, Gerhard NK, Fugl M, Hegedus L. Thyroid volume measured by ultrasonography and CT. Acta Radiol 43:269-274, 2002.
- 94.
Bonnema SJ, Andersen PB, Knudsen DU, Hegedus L. MR imaging of large multinodular goiters: observer agreement on volume vs observer disagreement on dimensions of the involved trachea. AJR Am J Roentgenol 179:259-266, 2002.
- 95.
Schlogl S, Werner E, Lassmann M, Terekhova J, Muffert S, Seybold S, Reiners C. The use of three-dimensional ultrasound for thyroid volumetry. Thyroid 11:569-574, 2001.
- 96.
Rivo-Vázquez Á, Rodríguez-Lorenzo Á, Rivo-Vázquez JE, Páramo-Fernández C, García-Lorenzo F, Pardellas-Rivera H, Casal-Núñez JE, Gil-Gil P. The use of ultrasound elastography in the assessment of malignancy risk in thyroid nodules and multinodular Clin Endocrinol (Oxf). 79(6):887-91, 2013.
- 97.
Hays MT, Wesselossky B. Simultaneous measurement of thyroidal trapping (99 mTcO4-) and binding (¹³¹I-): clinical and experimental studies in man. J Nucl Med 14:785-792, 1973.
- 98.
Arnold JE. Pinsky S. Comparison of 99 mTc and ¹²³I for thyroid imaging. J Nucl Med 17:261-267, 1976.
- 99.
Dige-Petersen H, Kroon S, Vadstrup S, Andersen ML, Roy-Poulsen NO. A comparison of 99Tc and ¹²³I scintigraphy in nodular thyroid disorders. Eur J Nucl Med 3:1-4, 1978.
- 100.
Ryo UY, Vaidya PV, Schneider AB, Bekerman C, Pinsky SM. Thyroid imaging agents: a comparison of I-123 and TC-99m pertechnetate. Radiology 148:819-822, 1983.
- 101.
Wesche MF, Tiel-Van Buul MM, Smits NJ, Wiersinga WM. Ultrasonographics vs. scintigraphic measurement of thyroid volume in patients referred for 131I therapy. Nucl Med Commun 19:341-346, 1998.
- 102.
Wanet PM, Sand A, Abramovici J. Physical and clinical evaluation of high-resolution thyroid pinhole tomography. J Nucl Med 37:2017-2020, 1996.
- 103.
Jennings A. Evaluation of substernal goiters using computed tomography and MR imaging. Endocrinol Metab Clin North Am 30:401-414, 2001.
- 104.
Belardinlli L, Gualdi G, Ceroni L, Guadalaxara A, Polettini E, Pappalardo G. Comparison between computed tomography and magnetic resonance data and pathologic findings in substernal goiters. Int Surg 80:65-69, 1995.
- 105.
Hermans r, Bouillon R, LagaK, Delaere PR, Foer BD, Marchal G, Baer AL. Estimation of thyroid gland volume by spiral computed tomography. Eur Radiol. 7:214-216, 1997.
- 106.
Gullu S, Gurses MA, Baskal N, Uysal AR, Kamel AN, Erdogan G. Suppressive therapy with levothyroxine for euthyroid diffuse and nodular goiter. Endocr J 46:221-226, 1999
- 107.
Peters H, Hackel D, Schleusener H. Treatment of euthyroid struma. Comparable volume reduction with 400 micrograms iodine, 100 micrograms levothyroxine combined with 100 micrograms iodine or individually dosed levothyroxine. Med Klin 92:63-67, 1997.
- 108.
Schumm-Draeger PM. Drug therapy of goiter. Iodine, thyroid hormones or combined therapy. Z Gesamte Inn Med 48:592-598, 1993.
- 109.
Lima N, Knobel M, Cavaliere H, Sztejnsznajd, Tomimori E, Medeiros-Neto G. Levothyroxine suppressive therapy is partially effective in treating patients with benign, solid thyroid nodules and multinodular goiters. Thyroid 7:691-697, 1997.
- 110.
Wesche MF, Tiel-Van Buul MM, Lips P, Smits NJ, Wiersinga WM. A randomized trial comparing levothyroxine with radioactived iodine in the treatment of sporadic nontoxic goiter. J Clin Endocrinol Metab 86:998-1005, 2001.
- 111.
Papini E, Petrucni R, Guglielmi R, Panunzi C, Rinaldi R, Bacci V, Crescenzi A, Nardi F, Fabbrini R, Pecella CM. Long Term changes in Nodular Goiter: a 5 yr prospective randomized trial of L-T4 suppressive therapy for benign thyroid nodules. J Clin Endocr Metab 83:780-783, 1998.
- 112.
Zelmanovitz F, Genro S, Gross JL. Suppressive therapy with L-T4 for solitary thyroid nodules: a double blind controlled clinical study and cumulative Meta-Analysis. J Clin Endocr Metab 83:3881-3885, 1998.
- 113.
Berghout A, Wiersinga WM, Smits NJ, Touber JL. Interrelationships between age, thyroid volume, thyroid nodularity, and thyroid function in patients with sporadic non-toxic goiter. Am J Med 89:602-608,1995.
- 114.
Leese GP, Jung RT, Guthrie C, Waugh N, Browning MC. Morbidity in patients on L-thyroxine: a comparison of those with a normal TSH to those with a suppressed TSH. Clin Endocrinol (Oxf) 37:500-503, 1992.
- 115.
Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab 81:4278-4289, 1996.
- 116.
Hegedüs L, Hansen BM, Knudsen N, Hansen JM: Reduction of size of thyroid with radioactive iodine in multinodualr nontoxic goitre. Brit Med J 297:661, 1988.
- 117.
Hamburger JI, Hamburger SW: Diagnosis and management of large toxic multinodular goiters. J Nucl Med 26:888, 1985.
- 118.
Kay TW, d´Emden MC, Andrews JT, Martin FI: Treatment of non-toxic multinodular goiter with radioactive iodine. Am J Med 84:19, 1988.
- 119.
Huysmans D, Hermus A, Edelbroek M, et al. Radioiodine for nontoxic multinodular goiter. Thyroid 7:235-239, 1997.
- 120.
Nygaard B, Hegedüs L, Gervil M, Hjalgrim H, Soe-Jensen G et al. Radioiodine treatment of multinodular non-toxic goitre. Br Med J 307:828, 1993.
- 121.
Danaci M, Veek CM, Notghi A, Merck MV, Padfield PL, Edwards CR. 131I radioiodine therapy for hyperthyroidism in patients with Graves´ disease, uninodular goiter and multinodular goiter. N Z Med J 101:784, 1988.
- 122.
Nygaard B, Hegedus L, Ulriksen P, Nielsen KG, Hansen JM. Radioiodine therapy for multinodular toxic goiter. Arch Intern Med 159:1364-1368, 1999.
- 123.
Aach R, Kissane J: Thyroid storm shortly after 131I therapy of a toxic multinodular goiter. Am J Med 52:786, 1972.
- 124.
Huysmans Ak, Hermus RM, Cortstens FAM, Barents JO, Kloppenborgh PWC. Large compressive goiters treated with radioiodine. Ann Int Med 121:757, 1994.
- 125.
Nygaard B, Hegedus L, Gervil M, Hjalgrim H, Soe-Jensen P, Hansen JM. Radioiodine treatment of multinodular non-toxic goitre. BMJ 307(6908):828-32, 1993.
- 126.
Nielsen VE, Bonnema SJ, Hegedus L. The effects of recombinant human thyrotropin, in normal subjects and patients with goitre. Clin Endocrinol (Oxf) 61(6)655-63, 2004.
- 127.
Nielsen VE, Bonnema SJ, Boel-Jorgensen H, Grupe P, Hegedus L. Stimulation with 0.3 mg recombinant human thyrotropin prior to iodine 131I therapy to improve the size reduction of benign nontoxic nodular goiter: a prospective randomized double-blind trial. Arch Intern Med 166(14):1476-82, 2006.
- 128.
Nieuwlaat Wa, Huysmans DA, van den Bosch HC, Sweep CG, Ross HA, Corstens FH, et al. Pretreatment with a single, low dose of recombinant human thyrotropin allows dose reduction of radioiodine therapy in patients with nodular goiter. J Clin Endocrinol Metab 3121-9, 2003.
- 129.
Weetman AP. Radioiodine treatment for benign thyroid diseases. Clin Endocrinol (Oxf) 66(6):757-64, 2007.
- 130.
Huysmans DA, Nieuwlaat WA, Erdtsieck RJ, Schellekens AP, Bus JW, Bravenboer B. Administration of a single low dose of recombinant human thyrotropin significantly enhances thyroid radioiodide uptake in nontoxic nodular goiter. J Clin Endocrinol Metab 85(10):3592-6, 2000.
- 131.
Albino CC, Mesa CO, Jr, Olandoski M, Ueda CE, Woellner LC, Goedert CA, et al. Recombinant human thyrotropin as adjuvant in the treatment of multinodular goiters with radioiodine. J Clin Endocrinol Metab 90(5):2775-80, 2005.
- 132.
Cohen O,m Ilany J, Hoffman C, Olchovsky D, Dabhi S, Karasik A. Low-dose recombinant human thyrotropin-aided radioiodine treatment of large, multinodular goiters in elderly patients. Eur J Endocrinol 154(2):243-52, 2006.
- 133.
Giusti M, Caorsi V, Mortara L, Caputo M, Monti E, Schiavo M, Bagnara MC, Minuto F, Bagnasco M. Long-term outcome after radioiodine therapy with adjuvant rhTSH treatment: comparison between patients with non-toxic and pre-toxic large multinodular goitre. Endocrine (2):221-9, 2014.
- 134.
Duick DS, Baskin HJ. Utility of recombinant human thyrotropin for augmentation of radioiodine uptake and treatment of nontoxic and toxic multinodular goiters. Endocr Pract 9(3):204-9, 2003.
- 135.
Duick DS, Baskin HJ. Significance of radioiodine uptake at 72 hours versus 24 hours after pretreatment with recombinant human thyrotropin for enhancement of radioiodine therapy in patients with symptomatic nontoxic multinodular goiter. Endocr Pract 10(3):253-60, 2004.
- 136.
Fast S, Hegedüs L, Pacini F, Pinchera A, Leung AM, Vaisman M, Reiners C, Wemeau JL, Huysmans DA, Harper W, Rachinsky I, de Souza HN, Castagna MG, Antonangeli L, Braverman LE, Corbo R, Düren C, Proust-Lemoine E, Marriott C, Driedger A, Grupe P, Watt T, Magner J, Purvis A, Graf H. Long-term efficacy of modified-release recombinant human thyrotropin augmented radioiodine therapy for benign multinodulargoiter: results from a multicenter, international, randomized, placebo-controlled, dose-selection study. 2014.
- 137.
Paz-Filho GJ, Mesa-Junior CO, Olandoski M, Woellner LC, Goedert CA, Boguszewski CL, et al. Effect of 30 mCi radioiodine on multinodular goiter previously treated with recombinant human thyroid stimulating hormone. Braz J Med Biol Res 40(12):1661-70, 2007.
- 138.
Nielsen VE, Bonnema SJ, Boel-Jorgensen H, Veje A, Hegedus L. Recombinant human thyrotropin markedly changes the 131I kinetics during 131I therapy of patients with nodular goiter: an evaluation by a randomized double-blinded trial. J Clin Endocrinol Metab 90(1):79-83, 2005.
- 139.
Niewlaat WA, Hermus AR, Sivro-Pmdelj F, Corstens FH, Huysmans DA. Pretreatment with recombinant human TSH changes the regional distribution of radioiodine on thyroid scintigrams of nodular goiters. J Clin Endocrinol Metab 86(11):5330-6, 2001.
- 140.
Giusti M, Cappi C, Santaniello B, Ceresola E, Augeri C, Lagasio C, et al. Safety and efficacy of administering 0.2 mg of recombinant human TSH for two consecutive days as an adjuvant to therapy with low radioiodine doses in elderly out-patients with large nontoxic multinodular goiter. Minerva Endocrinol 31(3):191-209, 2006.
- 141.
Bonnema SJ, Nielsen VE, Boel-Jorgensen H, Grupe P, Andersen PB, Bastholt L, et al. Improvement of goiter volume reduction after 0.3 mg recombinant human thyrotropin-stimulated radioiodine therapy in patients with a very large goiter: a double-blinded, randomized trial. J Clin Endocrinol Metab 92(9):3424-8, 2007.
- 142.
Silva MN, Rubio IG, Romao R, Gebrin EM, Buchpiguel C, Tomimori E, Camargo RYA, Cardia MS, Medeiros-Neto G. Administration of a single dose of recombinant human thyrotropin enhances the efficacy of radioiodine treatment of large compressive multinodular goitres. Clin Endocrinol (Oxf) 60(3):300-8, 2004.
- 143.
Paz-Filho G, Mesa C, Carvalho G, Goedert C, Graf H. Recombinant human TSH associated with radioiodine does not have further effects on thyroid volume and function after two years. Clin Endocrinol (Oxf) 2007 (Letter|).
- 144.
Nieuwlaat WA, Hermus AR, Ross HA, Buijs WC, Edelbroek MA, Bus JW, et al. Dosimetry of radioiodine therapy in patients with nodular goiter after pretreatment with a single, low dose or recombinant human thyroid-stimulating hormone. J Nucl Med 45(4):626-33, 2004.
- 145.
Nielsen VE, Bonnema SJ, Hegedus L. Transient goiter enlargement after administration of 0.3 mg of recombinant human thyrotrophin in patients with benign nontoxic nodular goiter: a randomized, double-blind, crossover trial. J Clin Endocrinol Metab 91(4):1317-22, 2006.
- 146.
Fast S., Nielsen VE, Grupe P, Boel-Jaegersen H, Bastholt L, Andersen PB, Bonnema SJ, Hegedus L. Prestimulation with recombinant human thyrotropin (rhTSH) improves the long-term outcome of radioiodine therapy for multinodular nontoxic goiter. J Clin Endocrinol Metab 98::2653-2660, 2012
- 147.
Cubas ER, Paz-Filho GJ, Olandoski M, Goedert CA, Woellner LC, Carvalho GA, Graf H. Recombinant human TSH increases the efficacy of a fixed activity of radioiodine for treatment of multinodular goitre. Int J Clin Pract 63:585-590, 2009.
- 148.
Romão R, Rubio IGS, Tomimori EK, Camargo RYA, Knobel M, Medeiros-Neto G. High prevalence of side effects after rhTSH-stimulated radioiodine treatment with 30 mCi in patients with multinodular goiter and subclinical / clinical hyperthyroidism. Thyroid 19:945-951, 2009.
- 149.
Cardia MS, Rubio IG, Medeiros-Neto G. Prolonged follow-up of multinodular goitre patients treated with radioiodine preceded or not by human recombinant TSH. Clin Endocrinol (Oxf) 64(4):474, 2006.
- 150.
Barca MF, Gruppi C, Oliveira MT, Romao R, Cardia MS, Rubio IGS, Knobel M, Medeiros-Neto G. Cardiovascular assessment of hyperthyroid patients with multinodular goiters before and after radioiodine treatment preceded by stimulation with recombinant human TSH. Endocrine 70:810-813, 2009.
- 151.
Nygaard B, Knudsen JH, Hedegus L, Scient AV, Hansen JE. Thyrotropin receptor antibodies and Graves´ disease, a side-effect of 131I treatment in patients with nontoxic goiter. J Clin Endocrinol Metab. 82(9):2926-30, 1997.
- 152.
Rubio IG, Perone BH, Silva MN, Knobel M, Medeiros-Neto G. Human recombinant TSH preceding a therapeutic dose of radioiodine for multinodular goiters has no significant effect in the surge of TSH-receptor and TPO antibodies. Thyroid 15(2):134-9, 2005.
- 153.
Medeiros-Neto G, Marui S, Knobel M. An outline concerning the potential use of recombinant human thyrotropin for improving radioiodine therapy of multinodular goiter. Endocrine 33:109-117, 2009.
- 154.
Fast S, Nielsen UE, Bonnema SJ, Hegedus L. Time to reconsider non- surgical therapy of benign non-toxic multinodular goiter: focus on recombinant human TSH augmented radioiodine therapy. Eur J Endocrinol 160:517-528, 2009.
- 155.
Magner J. Problems associated with the use of thyrogen in patients with a thyroid gland. N Engl J Med 359:1738-9, 2008.
- 156.
Fast S, Nielsen VE, Bonnema SJ, Hegedus L. Optimizing 131I uptake after rhTSH stimulation in Patients with multinodular goiter: evidence from a prospective double blind study. J Nucl Med 50:732-737, 2009.
- 157.
Bonnema SJ, Hegedus L, A 30 year perspective on radioiodine therapy of benign nontoxic multinodular goiter. Cur Op Endocr Metab Diab Obes. 16:379-384, 2009.
- 158.
Pacella CM,Mauri G, Achille G, Barbaro D, Bizzarri G, De Feo P, Di Stasio E, Esposito R, Gambelunghe G, Misischi I,Raggiunti B, Rago T, Patelli GL, D'Este S, Vitti P, Papini E. Outcomes and Risk Factors for Complications of Laser Ablation for Thyroid Nodules: A Multicenter Study on 1531 Patients. J Clin Endocrinol Metab. 100(10):3903-10, 2015.
- Review Evaluation and management of multinodular goiter.[Otolaryngol Clin North Am. 1996]Review Evaluation and management of multinodular goiter.Hurley DL, Gharib H. Otolaryngol Clin North Am. 1996 Aug; 29(4):527-40.
- High prevalence of side effects after recombinant human thyrotropin-stimulated radioiodine treatment with 30 mCi in patients with multinodular goiter and subclinical/clinical hyperthyroidism.[Thyroid. 2009]High prevalence of side effects after recombinant human thyrotropin-stimulated radioiodine treatment with 30 mCi in patients with multinodular goiter and subclinical/clinical hyperthyroidism.Romão R, Rubio IG, Tomimori EK, Camargo RY, Knobel M, Medeiros-Neto G. Thyroid. 2009 Sep; 19(9):945-51.
- Single, very low dose (0.03 mg) of recombinant human thyrotropin (rhTSH) effectively increases radioiodine uptake in the I-131 treatment of large nontoxic multinodular goiter.[Nucl Med Rev Cent East Eur. 2016]Single, very low dose (0.03 mg) of recombinant human thyrotropin (rhTSH) effectively increases radioiodine uptake in the I-131 treatment of large nontoxic multinodular goiter.Mojsak MN, Abdelrazek S, Szumowski P, Rogowski F, Sykała M, Kostecki J, Kociura-Sawicka A, Jurgilewicz D, Myśliwiec J. Nucl Med Rev Cent East Eur. 2016; 19(1):3-11.
- Review Recombinant human TSH and radioactive iodine therapy in the management of benign multinodular goiter.[Eur J Endocrinol. 2015]Review Recombinant human TSH and radioactive iodine therapy in the management of benign multinodular goiter.Graf H. Eur J Endocrinol. 2015 Feb; 172(2):R47-52. Epub 2014 Sep 4.
- Multinodular goiter management in Western Saudi Arabia.[Saudi Med J. 2005]Multinodular goiter management in Western Saudi Arabia.Qari FA. Saudi Med J. 2005 Mar; 26(3):438-41.
- Multinodular Goiter - EndotextMultinodular Goiter - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
